Fig. 12.1
Rest EKG performed in hospital
Conclusions: sinus rhythm, normal atrioventricular conduction, counterclockwise rotation on horizontal axis, low voltages on limb leads, and diffuse nonspecific alterations in repolarization
Routine Laboratory Tests
Complete blood count: normal
Cholesterol (total, HDL, LDL) and TG: normal
Hepatic function (GOT, GPT, γ-GGT, ALP, total bilirubin, direct and indirect bilirubin): normal
Thyroid function (TSH, FT3, FT4): normal
Renal function (creatinine, BUN): normal
Electrolytes (Na + , K + , Ca ++ , Mg ++ , Cl − ): normal
Fasting blood glucose: 78 m/dl (4.33 mmol/L)
Troponin I-hs: 0.16 ng/ml (n.v. < 0.055 ng/ml)
BNP: 1195 pg/ml (n.v. <100 pg/ml)
Inflammation index: VES 3 mm/h (n.v. < 27 mm/h), CRP 0.3 mg/dl (n.v. <0.6 ng/ml)
D-dimers: 213 ng/ml (n.v. <230 ng/ml)
Pulmonary Function Tests
No alteration in pulmonary volumes and, normal Tiffeneau index, with mild reduction in DLCO.
Normal D-dimers, showed at routine laboratory tests, ruled out pulmonary embolism. According to EKG bradyarrhythmias could be excluded and normal red blood cells count excluded the presence of anemia. Finally, COPD was excluded by pulmonary function tests.
The diagnosis of heart failure is now very likely according to symptoms, physical exam, chest radiography, and laboratory tests.
Which Are the Possible Causes of Heart Failure?
Myocardial disease
Coronary artery disease
Hypertension
Cardiomyopathy
Familial (hypertrophic, dilated, ARVD, restrictive, left ventricular non-compaction)
Myocarditis (infective, immune mediated, chemotherapy, cocaine, alcohol, heavy metals)
Endocrine/nutritional (pheochromocytoma, vitamin deficiency, hypophosphatemia, hypocalcemia)
Pregnancy
Infiltration (amyloidosis, malignancy)
Valvular heart disease
Mitral
Aortic
Tricuspid
Pulmonary
Pericardial disease
Constrictive pericarditis
Pericardial effusion
Congenital heart disease
Arrhythmias
Tachyarrhythmias (atrial, ventricular)
Bradyarrhythmias (sinus node dysfunction, atrioventricular block)
High-output states
Anemia
Sepsis
Thyrotoxicosis
Paget’s disease
Arteriovenous fistula
Volume overload
Renal failure
Iatrogenic (postoperative fluid infusion)
HF may be the final result of different pathological pathways. However, some possibilities can be excluded just considering medical history, physical examination, and EKG. Our patient was smoker, but did not have other risk factors for coronary artery disease (CAD); a diagnosis of ischemic cardiomyopathy was unlikely. A heart valvular disease was excluded by the physical examination. The patient had not any familiar history for genetic cardiomyopathies (ARVD, hypertrophic, non-compaction, dilated), albeit this is not an absolute exclusion criteria. The basal EKG did not show brady- or tachyarrhythmias and the patient did not complain palpitations. Finally, normal routine laboratory tests excluded anemia, thyrotoxicosis, renal failure, and sepsis.
Acute myocarditis remains a valid possibility (rapid onset of symptoms, presence of cough and dyspnea) and also pericarditis (atypical chest pain and low voltages, albeit in only limb leads, at basal EKG). In addition infiltrative cardiomyopathy cannot be excluded.
The next step was to record an echocardiogram in order to evaluate function and morphology of the left ventricle.
Echocardiography Results (Fig. 12.2a, b)
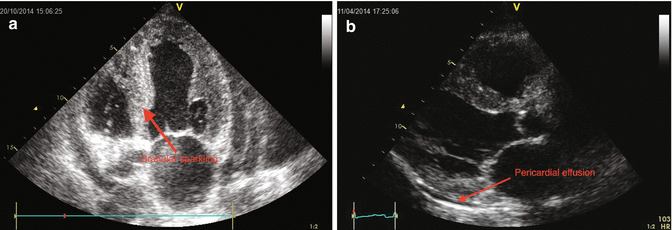
Fig. 12.2
(a, b) PLAX and apical 4-CH short axis showed left ventricular hypertrophy with “granular sparkling” appearance and pericardial effusion
Bi-atrial enlargement (LA diameter M-mode = 50 cm; area 4c = 26 cm2, RA area 4c = 20 cm2). Small size of the left ventricle (iLVEDV 44 ml/m2), increased wall thickness (IVSDd = 1.4 cm) with granular sparkling appearance (particularly evident at the level of the inter ventricular septum) and reduced systolic function (ejection fraction with Simpson’s rule 0.45). Normal size of the right ventricle, with increased free wall thickness and reduced systolic function (TAPSE 11 mm). Inter atrial septum thickening.
Mitral valve leaflets are thickened with mild regurgitation.
Tricuspid valve leaflets are also thickened with mild regurgitation and normal systolic pressure gradient (PASP = 30 mmHg).
Normal aortic and pulmonic valves.
The inferior vena cava is slightly dilated (26 mm). There is less than 50% inspiratory collapse of the IVC.
Small circumferential pericardial effusion, not hemodynamically significant.
Restrictive diastolic pattern with increased filling pressure (E/A 4, E/E’ 29, E dec time 147 m/sec).
Conclusion : mild biventricular systolic dysfunction. Biventricular wall thicknesses with granular sparkling aspect. Bi-atrial enlargement. Severe left ventricular diastolic dysfunction. Mild pleural effusion. Not significant valve regurgitation or gradients.
The echocardiography findings were compatible with hypertrophic/hypertensive or infiltrative cardiomyopathy.
The patient had no history of hypertension and arterial pressure values were normal during physical examination, so a hypertensive cardiomyopathy was unlikely.
Distinguishing between hypertrophic and infiltrative, only by echocardiography, is an easy task. The ventricle involvement is usually concentric in the infiltrative forms and eccentric in the hypertrophic forms. The presence of dynamic obstruction of left ventricular output tract is more frequent in hypertrophic forms. A pericardial effusion and the typical pattern of granular sparkling of the myocardium are suggestive of an infiltrative form. A severe diastolic dysfunction (grade III) may be found in both diseases.
In our case the typical echocardiographic pattern (wall thickening with granular sparkling aspect and severe diastolic dysfunction) in addition to low electrocardiographic limb lead voltages makes an infiltrative cardiac disease more likely.
Cardiac RMN
In order to acquire further diagnostic elements about ventricle morphology of the ventricles, a cardiac RMN was performed (Fig. 12.3a, b).
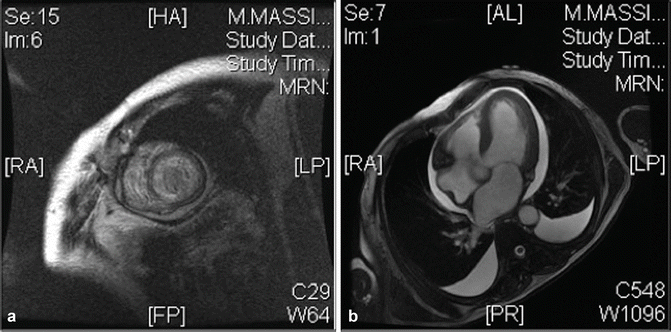

Fig. 12.3
(a, b) Magnetic resonance showed areas of delayed enhancement (DA), diagnostic for amyloidosis and pleuropericardial effusion
[…] bi-ventricular parietal hypertrophy and systolic dysfunction (LVEF 41%, RVEF 47%). Bi-atrial enlargement (left > right). Mild mitral and tricuspid regurgitation. No myocardial edema. Post contrastographic diffuse subendocardial late enhancement. Moderate circumferential pericardial effusion.
Conclusion: pattern suggestive of cardiac amyloidosis
There are three main types of cardiac amyloidosis: AL-related amyloidosis, ATTRm amyloidosis, and ATTRwt amyloidosis. According to familial history negative for cardiovascular disease, the patient’s age, and epidemiology, the most likely diagnosis is AL-related amyloidosis.
The next step to confirm this hypothesis is to evaluate the presence of a high production of immunoglobulin light chains. For this purpose we performed a serum electrophoresis, which showed a monoclonal peak in the gamma zone immunofixation that demonstrates an increase of light-chain quantity in blood and urine (Fig. 12.4).
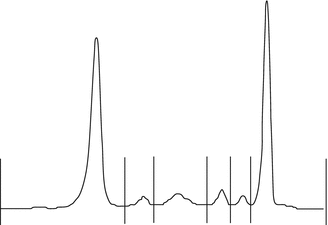

Fig. 12.4
Monoclonal proliferation at serum electrophoresis
Bone marrow biopsy (BOM) confirmed the diagnosis of multiple myeloma (MM), and finally, abdomen fat biopsy confirmed the diagnosis of amyloidosis.
Final Diagnosis
Cardiac-acquired monoclonal immunoglobulin light-chain (AL) amyloidosis.
The patient was treated with i.v. diuretic with consequent progressive resolution of the peripheral edema, lung congestion, and improvement of dyspnea and NYHA functional class. Because of low potassium serum level, an oral potassium-sparing diuretic was added. Other heart failure-specific drugs (that were introduced during diagnostic work up) such as renin-angiotensin-aldosterone antagonist (ACE-I and ARBs) and beta-blockers were interrupted according to scientific evidences.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


