Chapter 2 Cardiac activation
After reading this chapter, you should:
REQUIREMENTS FOR AN EFFECTIVE HEART
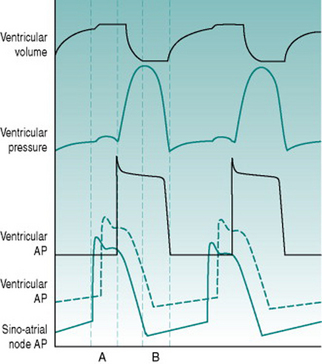
As a foundation from which to explore the responses of the circulation to exercise and environment, it is worthwhile to review the characteristics that the heart requires in order to fulfil its role as a pump. At least most of the material covered in this first section is probably familiar to you from earlier courses, but it may be helpful to have it set out again. As you read through the first section of this chapter, use the simulated records in Figure 2.1 to remind yourself of the sequences of electrical and mechanical events that make up the cardiac cycle.
Rhythmic excitation
For a muscular pump to provide continuous blood flow through the circulation there must be a system that guarantees generation of muscle action potentials at regular intervals. The normal rhythm generator or pacemaker is a small group of cells that lies high in the wall of the right atrium, constituting the so-called sinoatrial node. The membranes of these cells have an unusually high sodium conductance and contain metabolic pumps that progressively reduce potassium conductance. These two properties together result in the membrane potential depolarizing gradually towards zero in what is termed the pacemaker potential, rather than them having a stable resting membrane potential of around −70 mV as is characteristic of the normal cardiac muscle cells. Since the threshold membrane potential for action potential initiation is around −40 mV, the pacemaker cells fire an action potential as soon as they depolarize to this threshold. As in any excitable cell, the action potential activates a delayed potassium efflux and this repolarizes the cells to around −70 mV but, once these voltage-gated potassium channels close, the membrane starts to depolarize once more and initiates another action potential.
The period between successive action potentials in a sinoatrial node cell at 37° C (98.5° F) is around 600 ms, giving an inherent frequency of cardiac excitation of about 100 beats/min. In the intact body, however, the slope of the pacemaker potential is subject to tonic influences by thoracic sympathetic nerves that increase the slope and speed up heart rate (tachycardia) and vagal parasympathetic nerves that reduce the slope and slow the heart down (bradycardia). In a resting subject, both of these neural inputs are continuously active but the bradycardic effect of the parasympathetic input predominates, so that resting heart rate is normally less than 100 beats/min and typically around 65–75 beats/min.
Direction of ventricular pressure gradient
The ventricular chambers are elongated, with the outlets into the arterial system lying at their rostral ends adjacent to the A/V margin. Efficient blood ejection, therefore, requires progressive development of pressure from the ventricular apex towards the exit site, although the apical region is furthest away from the point of entry of action potentials through the A/V node. To achieve the appropriate sequence of ventricular activation, the ventricular end of the A/V node is connected to a branching system of large-diameter (that is, fast-conducting) cells forming the Purkinje system. This conducting system travels down each side of the interventricular septum, bypassing the more rostral regions of the ventricular muscle and directing excitation directly to the endocardial surface of the ventricular apices. Excitation then propagates back through the syncytium towards the A/V margin, pushing the ventricular contents towards its arterial exit points. The efficiency of ejection is further enhanced by the fact that the muscle making up the outer ventricular walls is arranged spirally, so that when it contracts it produces a wringing movement.
Guaranteed time for filling
Existence of a finite relaxation period is a prerequisite for nutritional perfusion of the ventricular myocardium. The coronary arteries that supply the heart arise from the aorta and so the pressure gradient responsible for coronary blood flow is essentially identical to that generated by the left ventricle. Because ventricular ejection must involve at least equivalent pressure being developed within the left ventricular wall, the left coronary arteries are mechanically occluded during the ejection phase of the cardiac cycle (termed systole) and nutritional perfusion can occur only during the relaxation phase (termed diastole). As will be discussed in Chapter 8 (p. 95), coronary perfusion of the right ventricle is more continuous, since the ventricular pressure developed there is much lower than systemic arterial pressure, and so the vasculature is perfused during systole as well as diastole.
Coordination of right and left outputs
Since the systemic and pulmonary circulations are in series, they must each pump identical volumes of blood per unit time. It is, therefore, essential that both ventricles eject blood simultaneously. This coordinated timing is ensured by the fact that the Purkinje system conveys action potentials simultaneously to the apices of both ventricles. Even with exact timing, however, the left ventricle needs to generate a far greater ejection pressure than the right ventricle in order to eject the same volume of blood, because the systemic vasculature has a much higher resistance than does the pulmonary vasculature. To provide the extra degree of pressurization, the left ventricle is wrapped with an additional spiral layer of muscle.