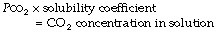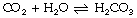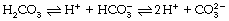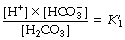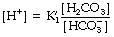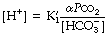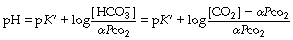Chapter 10 Carbon dioxide
 Most of the carbon dioxide carried in blood is in the form of bicarbonate, production and breakdown of which is catalysed by the enzyme carbonic anhydrase.
Most of the carbon dioxide carried in blood is in the form of bicarbonate, production and breakdown of which is catalysed by the enzyme carbonic anhydrase. Formation of bicarbonate is enhanced by the buffering of hydrogen ions by haemoglobin, and by active removal of bicarbonate ions from the red blood cell by Band 3 protein.
Formation of bicarbonate is enhanced by the buffering of hydrogen ions by haemoglobin, and by active removal of bicarbonate ions from the red blood cell by Band 3 protein. Smaller amounts of carbon dioxide are carried in solution in plasma, as carbonic acid, or as carbamino compounds formed with plasma proteins and haemoglobin.
Smaller amounts of carbon dioxide are carried in solution in plasma, as carbonic acid, or as carbamino compounds formed with plasma proteins and haemoglobin.Carbon dioxide is the end-product of aerobic metabolism and is produced almost entirely in the mitochondria where the Pco2 is highest. From its point of origin, there are a series of partial pressure gradients as carbon dioxide passes through the cytoplasm and the extracellular fluid into the blood. In the lungs, the Pco2 of the blood entering the pulmonary capillaries is normally higher than the alveolar Pco2, and therefore carbon dioxide diffuses from the blood into the alveolar gas, where a dynamic equilibrium is established. The equilibrium concentration equals the ratio between carbon dioxide output and alveolar ventilation (page 139). Blood leaving the alveoli has, for practical purposes, the same Pco2 as alveolar gas, and arterial blood Pco2 is usually very close to ‘ideal’ alveolar Pco2.
Abnormal levels of arterial Pco2 occur in a number of pathological states and have many important physiological effects throughout the body, some as a result of changes in pH, and these are discussed in Chapter 23. Fundamental to all problems relating to Pco2 is the mechanism by which carbon dioxide is carried in the blood.1
Carriage of Carbon Dioxide in Blood
In Physical Solution
Carbon dioxide is moderately soluble in water. According to Henry’s law of solubility:
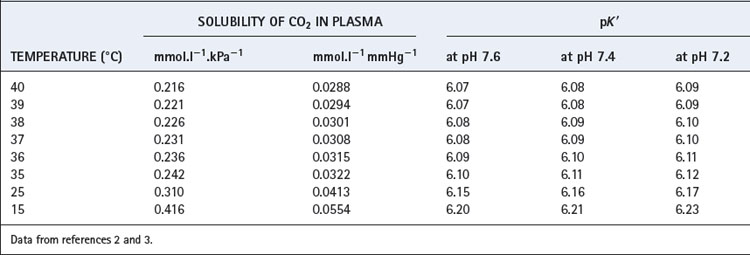
The solubility coefficient of carbon dioxide (α) is expressed in units of mmol.l−1 kPa−1 (or mmol.l−1.mmHg−1). The value depends on temperature, and examples are listed in Table 10.1. The contribution of dissolved carbon dioxide to the total carriage of the gas in blood is shown in Table 10.2.
As Carbonic Acid
In solution, carbon dioxide hydrates to form carbonic acid:
The equilibrium of this reaction is far to the left under physiological conditions. Published work shows some disagreement on the value of the equilibrium constant, but it seems likely that less than 1% of the molecules of carbon dioxide are in the form of carbonic acid. There is a very misleading medical convention by which both forms of carbon dioxide in equation (2) are sometimes shown as carbonic acid. Thus the term H2CO3 may, in some situations, mean the total concentrations of dissolved CO2 and H2CO3; to avoid confusion it is preferable to use αPco2 as in equation (7) below. This does not apply to equations (4) and (5) below, where H2CO3 has its correct meaning.
Carbonic anhydrase.4,5 The reaction of carbon dioxide with water (equation 2) is non-ionic and slow, requiring a period of minutes for equilibrium to be attained. This would be far too slow for the time available for gas exchange in pulmonary and systemic capillaries if the reaction were not catalysed in both directions by the enzyme carbonic anhydrase (CA). In addition to its role in the respiratory transport of carbon dioxide, CA plays a fundamental role in many body tissues, for example the generation of hydrogen and bicarbonate ions in secretory organs including the stomach and kidney, and the intracellular transfer of carbon dioxide within both skeletal and cardiac muscle. In mammals there are now 16 isozymes of CA identified, of which two are involved in blood carbon dioxide transport.
Red blood cells (RBCs) contain large amounts of CA II, one of the fastest enzymes known, whilst CA IV is a membrane bound isozyme present in pulmonary capillaries. There is no CA activity in plasma. Carbonic anhydrase is a zinc-containing enzyme of low molecular weight, and there is now extensive knowledge of the molecular mechanisms of CA.5,6 First, the zinc atom hydrolyses water to a reactive Zn–OH− species, whilst a nearby histidine residue acts as a ‘proton shuttle’, removing the H+ from the metal-ion centre and transferring it to any buffer molecules near the enzyme. Carbon dioxide then combines with the Zn–OH− species and the 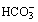 formed rapidly dissociates from the zinc atom. The maximal rate of catalysis is determined by the buffering power in the vicinity of the enzyme, as the speed of the enzyme reactions are so fast that its kinetics are determined mostly by the ability of the surrounding buffers to provide/remove H+ ions to/from the enzyme.
formed rapidly dissociates from the zinc atom. The maximal rate of catalysis is determined by the buffering power in the vicinity of the enzyme, as the speed of the enzyme reactions are so fast that its kinetics are determined mostly by the ability of the surrounding buffers to provide/remove H+ ions to/from the enzyme.
As Bicarbonate Ion
According to the law of mass action:
where K1′ is the equilibrium constant of the first dissociation. The subscript 1 indicates that it is the first dissociation, and the prime indicates that we are dealing with concentrations rather than the more correct thermodynamic activities.
Rearrangement of equation (4) gives the following:
The left-hand side is the hydrogen ion concentration, and this equation is the non-logarithmic form of the Henderson–Hasselbalch equation.7 The concentration of carbonic acid cannot be measured and the equation may be modified by replacing this term with the total concentration of dissolved CO2 and H2CO3, most conveniently quantified as αPco2 as described above. The equation now takes the form:
The equation is now in a useful form and permits the direct relation of plasma hydrogen ion concentration, Pco2 and bicarbonate concentration, all quantities that can be measured. The value of K′ cannot be derived theoretically and is determined experimentally by simultaneous measurements of the three variables. Under normal physiological conditions, if [H+] is in nmol.l−1, Pco2 in kPa, and [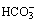 ] in mmol.l−1, the value of the combined parameter (αK′) is about 180. If Pco2 is in mmHg, the value of the parameter is 24.
] in mmol.l−1, the value of the combined parameter (αK′) is about 180. If Pco2 is in mmHg, the value of the parameter is 24.
Most people prefer to use the pH scale and so follow the approach described by Hasselbalch in 1916 and take logarithms of the reciprocal of each term in equation (6) with the following familiar result:8
where pK′ has an experimentally derived value of the order of 6.1, but varies with temperature and pH (see Table 10.1). ‘[CO2]’ refers to the total concentration of carbon dioxide in all forms (dissolved CO2, H2CO3 and bicarbonate) in plasma and not in whole blood.
Carbamino Carriage
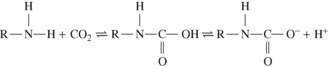
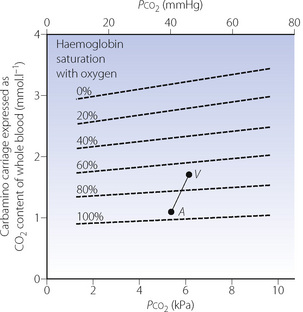
Carbamino carriage and haemoglobin. Only very small quantities of carbon dioxide are carried in carbamino compounds with plasma proteins. Almost all is carried by haemoglobin, and reduced haemoglobin is about 3.5 times as effective as oxyhaemoglobin (Figure 10.1), this being a major component of the Haldane effect (see below). Carbon dioxide binds to α-amino groups at the ends of both the α- and β-chains of haemoglobin. Earlier studies of CO2-haemoglobin reactions using free haemoglobin solution overestimated the magnitude of carbamino binding with haemoglobin, as later work showed that 2,3-diphosphoglycerate (2,3-DPG) present in vivo antagonises the binding of CO2 with haemoglobin. This antagonism results from direct competition between CO2 and 2,3-DPG for the end-terminal valine of the β-chain of haemoglobin, an effect that is not observed on the α-chains.
The Haldane effect. This is the difference in the quantity of carbon dioxide carried, at constant Pco2, in oxygenated and deoxygenated blood (Figure 10.2). Although the amount of carbon dioxide carried in the blood by carbamino carriage is small, the difference between the amount carried in venous and arterial blood is about a third of the total arterial/venous difference (Table 10.2). This therefore accounts for the major part of the Haldane effect, the remainder being due to the increased buffering capacity of reduced haemoglobin, which is discussed below. When the Haldane effect was described by Christiansen, Douglas & Haldane in 1914, they believed that the whole effect was due to altered buffering capacity:9 carbamino carriage was not demonstrated until much later (1934).10
Effect of Buffering Power of Proteins on Carbon Dioxide Carriage
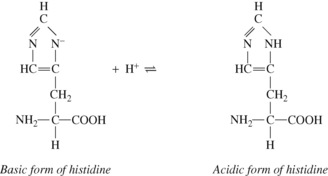
Amino and carboxyl groups concerned in peptide linkages have no buffering power. Neither have most side chain groups (e.g. in lysine and glutamic acid) because their pK values are far removed from the physiological range of pH. In contrast is the imidazole group of the amino acid histidine, which is almost the only amino acid to be an effective buffer in the normal range of pH. Imidazole groups constitute the major part of the considerable buffering power of haemoglobin, each tetramer containing 38 histidine residues. The buffering power of plasma proteins is less and is directly proportional to their histidine content.
The four haem groups of a molecule of haemoglobin are attached to the corresponding four amino acid chains at one of the histidine residues on each chain (page 188), and the dissociation constant of the imidazole groups of these four histidine residues is strongly influenced by the state of oxygenation of the haem. Reduction causes the corresponding imidazole group to become more basic. The converse is also true: in the acidic form of the imidazole group of the histidine, the strength of the oxygen bond is weakened. Each reaction is of great physiological interest and both effects were noticed many decades before their mechanisms were elucidated.
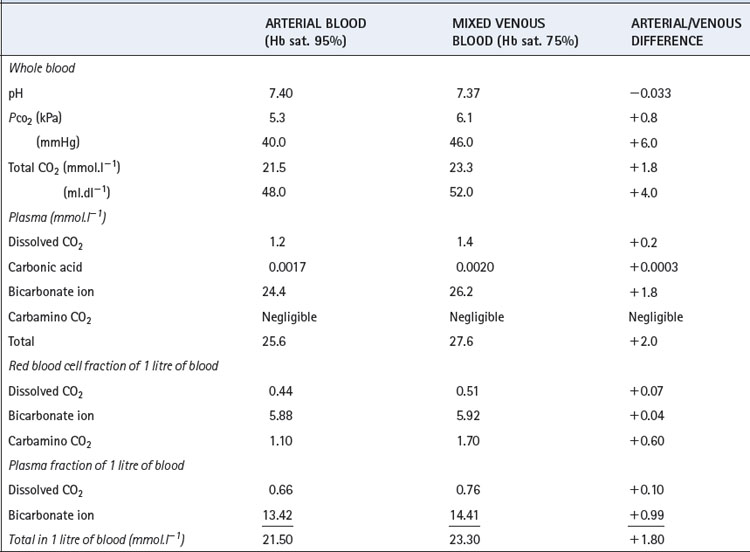
Total deoxygenation of the haemoglobin in blood would raise the pH by about 0.03 if the Pco2 were held constant at 5.3 kPa (40 mmHg), and this would correspond roughly to the addition of 3 mmol of base to 1 litre of blood. The normal degree of desaturation in the course of the change from arterial to venous blood is about 25%, corresponding to a pH increase of about 0.007 if Pco2 remains constant. In fact, Pco2 rises by about 0.8 kPa (6 mmHg), which would cause a decrease of pH of 0.040 if the oxygen saturation were to remain the same. The combination of an increase of Pco2 of 0.8 kPa and a decrease of saturation of 25% thus results in a fall of pH of 0.033 (Table 10.2).
Distribution of Carbon Dioxide within the Blood
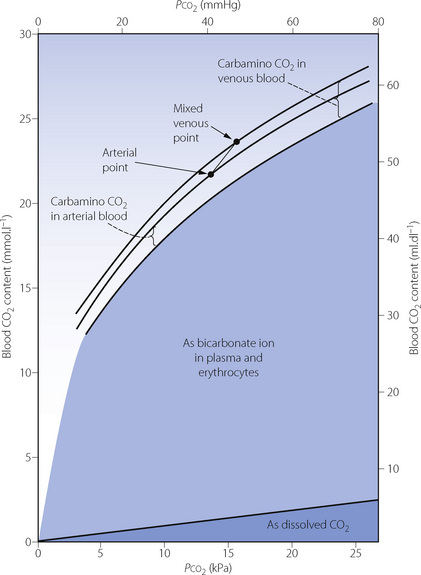
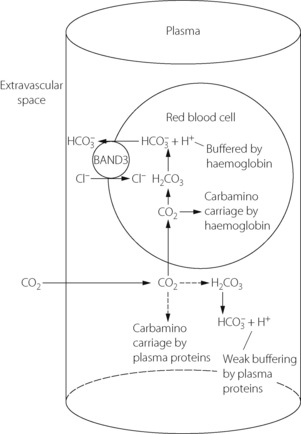
Table 10.2 shows the forms in which carbon dioxide is carried in normal arterial and mixed venous blood. Although the amount carried in solution is small, most of the carbon dioxide enters and leaves the blood as CO2 itself (Figure 10.3). Within the plasma there is little chemical combination of carbon dioxide, for three reasons. First, there is no carbonic anhydrase in plasma and therefore carbonic acid is formed only very slowly. Secondly, there is little buffering power in plasma to promote the dissociation of carbonic acid. Thirdly, the formation of carbamino compounds by plasma proteins is not great, and must be almost identical for arterial and venous blood.
Carbon dioxide can, however, diffuse freely into the RBC, where two courses are open. First, increasing intracellular Pco2 will increase carbamino carriage of CO2 by haemoglobin, an effect greatly enhanced by the fall in oxygen saturation which is likely to be occurring at the same time (Figure 10.1). The second course is hydration and dissociation of CO2 to produce hydrogen and bicarbonate ions, facilitated by the presence of carbonic anhydrase in the RBC. However, accumulation of intracellular hydrogen and bicarbonate ions will quickly tip the equilibrium of the reaction against further dissociation of carbonic acid, a situation that is avoided in the RBC by two mechanisms:
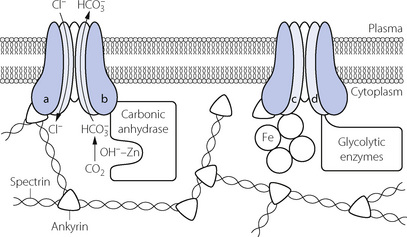
 RBC cytoskeleton. The cytoplasmic domain of band 3 acts as an anchoring site for many of the proteins involved in the maintenance of cell shape and membrane stability such as ankyrin and spectrin. A genetically engineered deficiency of band 3 in animals results in small, fragile, spherical RBCs,12 and in humans an inherited defect of band 3 is responsible for hereditary spherocytosis in which RBCs become spheroidal in shape and fragile.15,16 RBC shape and deformability are now known to be important in oxygen transport in the capillaries (page 148) and it is possible that band 3 is involved in bringing about these shape changes.
RBC cytoskeleton. The cytoplasmic domain of band 3 acts as an anchoring site for many of the proteins involved in the maintenance of cell shape and membrane stability such as ankyrin and spectrin. A genetically engineered deficiency of band 3 in animals results in small, fragile, spherical RBCs,12 and in humans an inherited defect of band 3 is responsible for hereditary spherocytosis in which RBCs become spheroidal in shape and fragile.15,16 RBC shape and deformability are now known to be important in oxygen transport in the capillaries (page 148) and it is possible that band 3 is involved in bringing about these shape changes. Carbonic anhydrase. Band 3 is also closely associated with carbonic anhydrase, and the protein complex formed is believed to act as a metabolon, a term describing the channelling of a substrate directly between proteins that catalyse sequential reactions in a metabolic pathway.13 In this case the substrate is bicarbonate, which after its formation by CA is transferred directly to band 3, which rapidly exports it from the cell.
Carbonic anhydrase. Band 3 is also closely associated with carbonic anhydrase, and the protein complex formed is believed to act as a metabolon, a term describing the channelling of a substrate directly between proteins that catalyse sequential reactions in a metabolic pathway.13 In this case the substrate is bicarbonate, which after its formation by CA is transferred directly to band 3, which rapidly exports it from the cell. Haemoglobin. Band 3 is also associated with haemoglobin, with which it is believed to form another metabolon system exporting nitric oxide derived nitrosothiols, possibly to regulate capillary blood flow and oxygen release from haemoglobin (page 195).
Haemoglobin. Band 3 is also associated with haemoglobin, with which it is believed to form another metabolon system exporting nitric oxide derived nitrosothiols, possibly to regulate capillary blood flow and oxygen release from haemoglobin (page 195). Glycolytic enzymes. Some of the enzymes involved in glycolysis (page 198), including glyceraldehyde-3-phosphate dehydrogenase, phosphofructokinase and aldolase, are bound to band 3; the functional significance of this is unknown.
Glycolytic enzymes. Some of the enzymes involved in glycolysis (page 198), including glyceraldehyde-3-phosphate dehydrogenase, phosphofructokinase and aldolase, are bound to band 3; the functional significance of this is unknown.