Figure 17–1.
Excitation-contraction coupling in the heart. With the advent of an action potential, L-ype Ca2+ channels are activated resulting in the influx of Ca2+. This influx of Ca2+ activates the adjacent RyR2 resulting in outpouring of Ca2+ stored in the sarcoplasmic reticulum (SR) into the cytosol. The release of Ca2+ activates the contractile proteins initiating systole. The increased cytosolic Ca2+ inactivates L-type Ca2+ channels, activates NCX (causing efflux of Ca2+) and activates SERCA2a (causing reuptake of Ca2+ into SR). This results in the termination of systole and the onset of diastolic phase. I Ca,L L-type Ca2+ current, NCX Na+/Ca2+-exchanger, PLN Phospholamban, RyR2 Ryanodine receptor type 2, SERCA2a Sarco/endoplasmic reticulum Ca2+‑ATPase, TnC Troponin C (Modified from Wehrens et al. [21]. With kind permission from Future Medicine Ltd)
Depolarization of the cardiomyocyte membrane leads to activation of voltage-gated L-type Ca2+ channels (LTCC) located in plasma membrane invaginations called transverse or T-tubules (see Fig. 17.1). Entry of Ca2+ through LTCCs triggers a much greater release of Ca2+ from the SR via the RyR2 intracellular Ca2+ release channels, which is known as Ca2+-induced Ca2+ release (CICR) [23]. For relaxation to occur during diastole, Ca2+ is extruded from the cytoplasm via a specialized Ca2+ pump known as the SR Ca2+ ATPase (SERCA2a). SERCA2a activity is regulated by phospholamban, which inhibits SR Ca2+ uptake in its non-phosphorylated form. Finally, cytosolic Ca2+ is also extruded from the cardiomyocyte via the electrogenic plasma membrane Na+/Ca2+ exchanger (NCX) [22, 24].
Advanced imaging techniques have revealed that cytosolic Ca2+ transients in cardiomyocytes represent the summation of 104 up to 106 localized, subcellular Ca2+ release events called Ca2+ sparks [25]. The CICR process amplifies and scales the intracellular Ca2+ signal via summation of functionally independent Ca2+ sparks [26]. Moreover, Ca2+ sparks are characterized by local refractoriness, suggesting that local SR Ca2+ release is independent of the duration of the LTCC-mediated Ca2+ influx [27]. It is estimated that about 100 or more RyR2 channels may become functionally coupled in a Ca2+ release unit in order to open and close simultaneously by the process of coupled gating [28]. On the other hand, Ca2+ release from the SR also results in local Ca2+ depletion in subcompartments of the SR Ca2+ stores [29]. Therefore, the intrinsic (e.g. channel composition) and extrinsic (e.g. regulation by extracellular signals) properties of RyR2 channel gating are an important determinant of the net Ca2+ concentration in the cytosol and the SR at any given time point during the cardiac contraction-relaxation cycle.
Cardiac Ryanodine Receptors
RyRs are comprised of four 560-kDa pore-forming subunits, which form tetrameric ion channels located on the SR membranes [30]. RyR2 is the predominant isoform in the heart and functions as the principal intracellular Ca2+ release channel during EC coupling [31]. Apart from a relatively small C-terminal pore-forming domain, approximately 90 % of the RyR2 N-terminal sequence forms the electron-dense cytosolic foot structure in the junctional space between the T-tubule and the SR (Fig. 17.2). This cytoplasmic RyR2 domain functions as a scaffold for modulating proteins, which regulate gating of the transmembrane Ca2+-conducting pore [24, 33, 34].
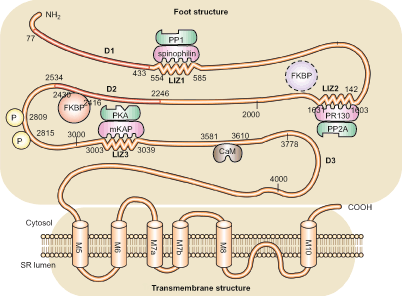

Figure 17–2.
Primary structure of the cardiac ryanodine receptor macromolecular complex. The primary structure of a cardiac ryanodine receptor subunit, with the binding domains of protein kinase A (PKA), protein phosphatases 1 and 2A (PP1, PP2A), calmodulin and FKBP12.6 indicated. PKA and PP1/PP2A are bound to RyR2 via their specific adaptor proteins. The domains highlighted in red (77–433, 1724–2534) as well as the transmembrane domains correspond to the three CPVT mutation hotspot regions (D1, D2 and D3). CaM calmodulin, FKBP FKBP12.6, LIZ leucine-isoleucine zipper, PKA protein kinase A, PP protein phosphatases, SR sarcoplasmic reticulum (From Macmillan Publishers Ltd: Yano et al. [32]. Reproduced with kind permission from Nature Publishing Group)
The Ryanodine Receptor Macromolecular Complex
A major regulatory subunit interacting with RyR2 Ca2+ release channels is the 12.6 kDa cytosolic FK506-binding protein (FKBP12.6), also known as calstabin2 [35, 36]. FKBP12.6, a peptidyl-prolyl cis–trans isomerase that tightly associates with RyR2 monomers, stabilizes the closed conformational state of RyR2 enabling the channel to close completely [37, 38]. Therefore, in the heart, an important physiologic role of FKBP12.6 appears to be inhibiting the RyR2 channel at low intracellular Ca2+ concentrations to ensure diastolic muscle relaxation and preventing detrimental effects of intracellular Ca2+ leak during diastole [13, 38]. In addition, FKBP12.6 plays a role in the orchestration of simultaneous RyR2 gating in Ca2+ release units comprised of functionally linked RyR2 [28, 39].
Other modulators associated with the large cytosolic RyR2 domain include calmodulin (CaM) [40], sorcin [41], the protein kinases A (PKA) [5], Ca2+/CaM-dependent protein kinase II (CaMKII) [42, 43], and the phosphatases, PP1 and PPA2A [2]. These kinases and phosphatases are believed to bind to RyR2 via specific anchoring proteins mAKAP, spinophilin and PR130, respectively (see Fig. 17.2). Conserved leucine/ isoleucine zipper (LIZ) motifs in the targeting proteins correspond to complementary LIZ domains in RyR2, allowing for highly localized RyR2 regulation.
RyRs also associate with proteins at the luminal surface within the SR membrane. Junctin and triadin are most likely involved in anchoring RyR2 to the SR membrane [44, 45]. Calsequestrin (CSQ) is the major Ca2+ binding protein in the SR and provides a high capacity intra-SR Ca2+ buffer [46]. Recent studies suggest that Ca2+-dependent conformational changes in CSQ modulate RyR2 channel activity and that dysfunctional luminal regulation of RyR2 may lead to cardiac arrhythmias (see below) [47]. Finally, we recently demonstrated that junctophilin-2 (JPH2) binds to RyR2 and mediates both physical and functional coupling between RyR2 and voltage-gated LTCC on the plasmalemma [48].
Regulation of RyR2
Phosphorylation
The maximal cardiac force development during systole (inotropy) can be enhanced by increasing the amplitude of the intracellular Ca2+ transient, which is dependent on the amount of intracellular Ca2+ released via RyR2 [49]. Upregulation of SR Ca2+ cycling can be achieved by phosphorylation of proteins involved in SR Ca2+ release and Ca2+ uptake pathways by cAMP-dependent PKA [5], CaMKII [43] and protein kinase C (PKC) [50]. A potent mechanism to increase cardiac contractility rapidly is mediated by stress-mediated activation of β(Beta)-adrenergic receptors by catecholamines resulting in increased intracellular cAMP synthesis and activation of PKA [51]. PKA phosphorylates both RyR2 to increase SR Ca2+ release,2 as well as phospholamban to enhance the activity of SERCA2A [52]. The net effect of β(Beta)-adrenergic receptor activation is amplification of the EC coupling gain such that for any given LTCC Ca2+ current more SR Ca2+ release is triggered [53].
Some studies have shown that phosphorylation by PKA increases RyR2 channel open probability by increasing the sensitivity to Ca2+-dependent activation [5, 38, 54, 55], although some studies indicate that PKA phosphorylation might not directly alter RyR2 open probability [56–58]. Although some studies suggest PKA might phosphorylate an additional residue on RyR2 in vitro [59], PKA phosphorylation of RyR2 is completely prevented in RyR2-S2808A knockin mice, in which serine 2808 is replaced by an alanine [60]. The transient nature of RyR2 activation by PKA phosphorylation in cells suggests powerful negative feedback mechanisms that prevent uncontrolled intracellular Ca2+ release. Indeed, RyR2 phosphorylation is locally regulated by protein phosphatases, which are targeted to the channel complex [2, 61]. Moreover, it was shown that the phosphodiesterase PDE4D3 associates with RyR2 limiting excessive phosphorylation of the channel, which has been shown to detrimentally affect the cardiac function in disease states of the heart [62].
RyR2 channels can also be phosphorylated by CaMKII, which phosphorylates a nearby site (serine 2814) on RyR2 and does not decrease the binding affinity of FKBP12.6 [43]. We demonstrated that genetic inhibition of S2814 in RyR2-S2814A mice inhibits the increase in RyR2 open probability following CaMKII phosphorylation of the channel [48]. CaMKII phosphorylation of RyR2 is believed to play an important role in the positive force-frequency relationship, as S2814A knock-in mice did not develop increased cardiac output at higher heart rates [63].
Redox Modulation
The open probability of RyR2 is not only modulated by phosphorylation but also by redox signaling [64–66]. RyR2 contains around 90 cysteine residues per monomer of which approximately 20 are in a reduced state and are potential targets for various redox modifications, including S-nitrosylation, S-glutathionylation, and disulfide crosslinking [67]. Experimental evidence suggests that redox modulation of RyR2 by NO, H2O2, nitroxyl, and hydroxyl radicals alter its activity in vitro [68, 69]. In general, oxidizing conditions increase the open probability of RyR2, whereas reducing reagents such as dithiothreitol reverse these effects [70–72]. However, prolonged exposure to high doses of oxidizing agents may cause irreversible and detrimental RyR activation [72, 73].
Although the effect of redox modulation of RyR2 is well established, the physiological role of endogenously produced oxidants is not. S-nitrosylation is an important reversible biological reaction involving nitric oxide in which nitrosyl groups are added to thiol residues. Out of the three known nitric oxide synthase (NOS) isoforms, cardiomyocytes express two constitutive NOS enzymes, neuronal NOS (nNOS) and endothelial NOS (eNOS). eNOS localizes to caveolae [74–76], where compartmentalization with β-adrenergic receptors and L-type Ca2+ channels [77] allows NO to inhibit β (beta)-adrenergic-induced inotropy [76, 78]. nNOS, however, is targeted to cardiac SR [79]. NO stimulation of SR Ca2+ release via RyR in vitro suggests that nNOS has an opposite, facilitative effect on contractility [67, 80]. This opposite effect is further evidenced by the observation that nNOS deficiency reduces inotropic response in contrast to eNOS deficiency that enhances contractility due to corresponding changes in SR Ca2+ release [81]. In addition to redox modulation by nitrosylation, cardiac SR is reported to have NADH oxidase as well as NOX2 oxidase (that utilizes NADPH instead of NADH) that regulate the RyR2 complex under physiological conditions [82–84].
Growing evidence suggests that redox modification of RyR2 may contribute to abnormal Ca2+ handling in disease states. S-nitrosylation (>3 sites per monomer) leads to reversible RyR activation [67]. In contrast, oxidation does not affect channel function up to a certain threshold (5–6 thiols per monomer), beyond which it leads to irreversible activation [67]. Moreover, oxidation of reactive thiols induces cross-linking between the subunits of RyR resulting in channel activation, which can be reversed by S-nitrosylation [65]. In addition, nNOS deficiency leads to an increase in reactive oxygen species (ROS) that may lead to oxidation of reactive thiols on RyR2 [85]. Thus, whereas S-nitrosylation seems to control the basal redox state of the channel, oxidation is a pathological process found in disease/stress states like HF [64].
Role of RyR2 in Arrhythmogenesis
Arrhythmias have a complex pathogenesis that include disorders of impulse formation or conduction or combinations thereof. Among the disorders of impulse formation, triggered activity in the form of early or delayed afterdepolarizations (EAD and DAD, respectively) is the underlying mechanisms for most ventricular arrhythmias. It is believed that diastolic opening of RyR2 may lead to the release of Ca2+ from the SR, which in turn activates the Na+/Ca2+-exchanger (NCX). Activation of NCX removes Ca2+ from the cytosol but also concurrently results in an inward Na+ current, which – if large enough – can trigger a DAD, extrasystole or even ventricular arrhythmia (Fig. 17.3) [90]. Such DAD-induced arrhythmias are found in patients with genetic mutations in RyR2 in the absence of structural heart disease (e.g., catecholaminergic polymorphic ventricular tachycardia, CPVT). In addition, conditions that cause [Ca2+]i overload such as HF and high digoxin levels, are also associated with DAD mediated ventricular arrhythmias [5, 6, 8, 91–94].
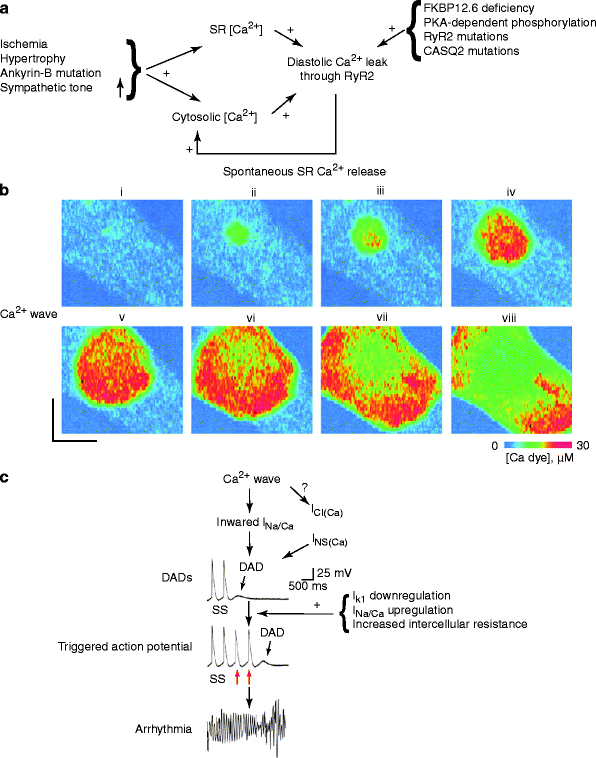

Figure 17–3.
Proposed scheme of events leading to delayed after-depolarizations (DAD) and triggered tachyarrhythmia. (a) Congenital (e.g., ankyrin-B mutation) and/or acquired factors (e.g., ischemia, hypertrophy, increased sympathetic tone) may cause a diastolic Ca2+ leak through ryanodine receptor channels type 2 (RyR2), resulting in localized and transient increases in [Ca2+]i in cardiomyocytes. (b) Representative series of images showing changes in [Ca2+]i during a Ca2+ wave in a single cardiomyocyte loaded with a Ca2+-sensitive fluorescent dye. Intracytosolic Ca2+ (i), focally elevated Ca2+ (ii) diffuses to adjacent junctional sarcoplasmic reticulum (SR), where it initiates more Ca2+ release events, resulting in a propagating Ca2+ wave (iii–viii). (c) The Ca2+ wave, through activation of Ca2+-sensitive inward currents, will depolarize the cardiomyocyte (DAD). In cardiomyocytes, the inward I(Na+/Ca2+) is the major candidate for the transient inward current underlying DADs, although the role of the Ca2+-activated Cl– current [I(Cl−(Ca2+))] and a Ca2+-sensitive nonspecific cation current [I(NS(Ca2+))] cannot be excluded. If of sufficient magnitude, the DAD will depolarize the cardiomyocyte above threshold resulting in a single or repetitive premature heartbeat (red arrows), which can trigger an arrhythmia. Downregulation of the inward rectifier potassium current (I K1 ), upregulation of I(Na+/Ca2+), or a slight increase in intercellular electrical resistance can promote the generation of DAD-triggered action potentials. S, stimulus (Modified from Rubart and Zipes [86], Mohler et al. [87], Qin et al. [88], Subramanian et al. [89]. With kind permission from American Society for Clinical Investigation)
Catecholaminergic Polymorphic Ventricular Tachycardia
CPVT is an inherited disorder characterized by exercise or stress-induced ventricular arrhythmias in patients with structurally normal heart (see also the CPVT chapter) [95]. The most common form is the autosomal-dominant CPVT type 1 (CPVT1), which is caused by mutations in the RYR2 gene. A less common form is CPVT type 2, which is autosomal-recessive and is caused by mutations in the CSQ2 gene, which encode CSQ isoform 2 (CSQ2) [3].
Over the past 10 years, >140 CPVT-linked mutations have been identified in the RYR2 gene (www.fsm.it/cardmoc). It is believed that RYR2 mutations cluster into three definable regions that are highly conserved across species [3]. These domains are often designated as the N-terminal domain (amino acids 1–600), the central domain (amino acids 2000–2500), and the C-terminal pore-forming domain (mutations clustered between amino acids 3800–4000 and 4500–5000) [3]. The vast majority of RYR2 mutations identified so far are missense mutations with less than 5 % being caused by small insertions/deletions [3].
Most studies have revealed that the functional consequence of RyR2 mutations is an increased open probability of the Ca2+ release channel [34]. The increased open probability of RyR2 is due to increased sensitivity of RyR2 to either luminal or cytosolic Ca2+ or both, such that minimal increases in either of the two Ca2+ stores lead to diastolic Ca2+ leak [38, 47]. Other studies have demonstrated that the gain-of-function channel phenotype can be unmasked following PKA phosphorylation [38]. These experimental findings correspond to the clinical observation that CPVT patients develop ventricular tachycardia in response to exercise and/or adrenergic stimulation [95–97]. Together, these observations support the view that the arrhythmias in CPVT1 need two substrates, a leaky RyR2 channel and a precipitant in the form of beta-adrenergic stimulation and/or increased luminal/cytosolic overload. This is because an increased diastolic Ca2+ leak at baseline will lead to depletion of SR Ca2+ stores thus restoring the Ca2+ release/leak to near-normal levels [98]. However, under conditions of beta-adrenergic stimulation and Ca2+ overload, enhanced SR Ca2+ loading will promote diastolic Ca2+ releases via mutant RyR2 channels leading to DADs and ventricular tachycardia [14].
Several mechanisms have been proposed to explain the gain-of-function defects found in CPVT mutant RyR2 channels. RyR2 has N-terminal and central domains that interact with each other to act as a switch that opens and closes the channel [99]. Zipping of the interacting domains facilitates channel closing, whereas unzipping promotes RyR2 opening. The presence of a CPVT mutation in either domain might weaken the interdomain interaction leading to domain unzipping and erroneous activation of RyR2 and diastolic Ca2+ leak [99]. This was evidenced in a mouse model with central domain CPVT1 mutation R2474S that showed aberrant unzipping of the domain switch regions, lowering of the threshold of luminal [Ca2+] for channel activation, sensitizing the channel to PKA-dependent phosphorylation, and CPVT [100]. In addition to the domain switch, central domain seems to have subdomain zipping too. A knock-in mouse model of mutation S2246L in the central domain revealed that an abnormally tight local subdomain-subdomain interaction might lead to defective interaction between the N-terminal and central domains [101].
Other studies have demonstrated that RyR2 mutations may impair the interaction between RyR2 and FKBP12.6, a channel-stabilizing subunit [38]. Several mutations in the N-terminal and central domain have been shown to lower FKBP12.6-RyR2 binding affinity [38, 90]. However, some studies have challenged this mechanism as some of these mutations were shown not to alter FKBP12.6 binding to RyR2 [47, 102]. On the other hand, a recent study linked domain unzipping and FKBP12.6 unbinding wherein PKA mediated hyperphosphorylation and dissociation of FKBP12.6 was associated with domain unzipping in failing hearts [103]. Although, the relative importance of the two mechanisms remains uncertain, but both domain unzipping and FKBP12.6 dissociation appear to promote pathogenic RyR2 gain-of-function associated with ventricular arrhythmias.
Although the pathogenic mechanisms underlying CSQ2 dysfunction in patients with CPVT-2 are beyond the scope of this chapter, it is thought that mutations in CSQ2 may lead to destabilization of the CICR mechanism by reducing effective Ca2+ buffering inside the SR [46], or causing altered interactions with the RyR2 channel complex leading to impaired RyR2 regulation by luminal Ca2+ [104]. Moreover, it has been suggested that RyR2 mutations might cause arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C) [105–107]. However, this association is generally doubted by most scientists in the field, and therefore, we will not discuss this condition in this chapter.
Drug-Induced Arrhythmias
It is well recognized that several drugs, in particular, some inotropic agents, can increase [Ca2+]i either by enhancing RyR2 activity or by inhibiting Na+/K+-ATPase [91, 92]. Among these, digoxin is the prototypical drug associated with [Ca2+]i overload-induced arrhythmias. Digitalis glycosides, including digoxin were the mainstay of HF therapy until the mid-1990s when the ‘Digitalis Investigation Group’ (DIG) trial showed a lack of reduction in mortality, and post-hoc analyses showing a possible increase in mortality in a subgroup of patients [108]. This increase in mortality is due to the narrow therapeutic range of digoxin. Because digoxin inhibits Na+/K+-ATPase, an excessive increase in [Na+]i can promote Ca2+ entry via the Na+/Ca2+ exchanger, which can eventually lead to SR Ca2+ overload [91]. Whereas increased SR Ca2+ content leads to enhanced systolic Ca2+ release and positive inotropy, diastolic SR Ca2+ leak might be detrimental due to the increase risk of ventricular arrhythmias as discussed above [91].
Other inotropes that include β (beta)1 agonist (epinephrine) activate the β (beta)-adrenoceptor/ adenylylcyclase/PKA cascade [92]. This results in production of cAMP and activation of intracellular PKA [92]. PKA in turn phosphorylates many proteins, including RyR2, which may promote diastolic Ca2+ leak, an increase in [Ca2+]i, and possibly activation of CaMKII, another important kinase that phosphorylates and activates RyR2 [5, 38, 43, 48, 54, 55]. Thus, most inotropic agents available till date have the potential to promote RyR2-mediated SR Ca2+ leak either directly by RyR2 phosphorylation or by increasing [Ca2+]i, setting the stage for increased arrhythmogenesis [91, 92].
Ventricular Arrhythmias in Failing Hearts
HF is a progressive disorder in which the heart is unable to pump blood commensurate to the needs of the body. HF is a leading cause of death with approximately half of the patients eventually dying due to lethal ventricular arrhythmias [109, 110]. HF is characterized by reduced cardiac contractility, which at the cellular level is related to reduced intracellular Ca2+ transients [8, 24, 111]. This reduction in Ca2+ transients has been attributed to both an increased SR Ca2+ leak (via RyR2) and reduction in SR Ca2+ loading (due to downregulation of SERCA2a activity) [5, 8, 111]. In early stages of HF, RyR2 phosphorylation due to β(Beta)-adrenergic receptor activation is believed to help maintain the systolic Ca2+ transient [5]. At more advanced stages of HF, however, chronic hyperphosphorylation of RyR2 will promote maladaptive remodeling characterized by sustained RyR2 leakiness associated with reduced SR Ca2+ stores and decreased systolic Ca2+ release [5, 6, 8, 93, 94].
Moreover, enhanced diastolic SR Ca2+ leak via RyR2 may induce arrhythmias via EADs or DADs [9, 112, 113]. As SR Ca2+ release depends on SR Ca2+ store loading, increased SR Ca2+ leak and reduced SR Ca2+ loading in HF loading has been predicted to reset the SR Ca2+ stores to a lower level that might intuitively reduce the diastolic Ca2+ leak [114, 115]. However, studies have revealed that in spite of reduced SR Ca2+ content, SR Ca2+ leak continues unabated in HF (‘Ca2+ overload paradox’) [8]. This increased diastolic Ca2+ leak in the presence of reduced SR Ca2+ content has been attributed to increased open probability of RyR2 due to either post-translational modification of the protein or changes in the composition of RyR2 macromolecular complex [8]. One of the most important modifiers of RyR2 function in HF is its increased phosphorylation by PKA and CaMKII, and reduced PP1 and PP2a activity [5, 6]. Activation of PKA and CaMKII can occur by many pathways, including chronic hyperadrenergic state found in patients with HF (PKA activation) [116], increased cytosolic Ca2+ (CaMKII activation) [6], and increased oxidative stress [8, 117, 118]. Increased oxidative stress can lead to oxidation of thiol residues on RyR2 or increase RyR2 phosphorylation by oxidative activation of CaMKII [64, 85]. Our recent studies have shown that inhibition of CaMKII phosphorylation of RyR2 reduces diastolic Ca2+ leak and ventricular arrhythmias in animal model of HF [48]. Thus, abnormal RyR2 function may contribute to ventricular arrhythmias in HF and could serve as a potential target for preventing sudden cardiac death in such patients [4–9, 48].
Duchenne Muscular Dystrophy
Duchenne muscular dystrophy (DMD) is the most common muscular dystrophy with an incidence of 1 in 3,500 male births [119]. Overall, a quarter of patients die from cardiac causes, half of which are due to sudden cardiac death [119]. The dystrophin gene codes for dystrophin protein and dystrophin-associated glycoproteins provide a structural link between the myocytes cytoskeleton and extracellular matrix [120]. In DMD, due to the absence of dystrophin, there is fragility of cell membrane, which results in abnormal stress-induced entry of Ca2+ into the cells leading to diastolic SR Ca2+ leak via RyR2 [121]. This was further confirmed in animal studies wherein diastolic Ca2+ release events and VT could be suppressed by pharmacological inhibition of RyR2 [16, 121]. DMD is one of many cardiac conditions that do not have a primary RyR2 disorder but have a higher RyR2-mediated SR Ca2+ leak. This highlights the central role of RyR2 in arrhythmogenesis not only in DMD but in other types of cardiomyopathies.
Ryanodine Receptors as a Therapeutic Target
Based on the discussion above, RyR2 plays an important role in the pathogenesis of atrial and ventricular arrhythmias arising from both genetically and acquired cardiac conditions [48, 122–126]. At present, there is no FDA approved medication that selectively target RyR2. Class II anti-arrhythmic agents (β (beta)-blockers) affect RyR2 function indirectly by reducing the sympathetic drive that drives RyR2 phosphorylation [116]. This class of drugs is also modestly effective in patients with CPVT, who typically still require ICD implantation once they have experienced syncope [95, 96]. There are efforts ongoing to develop drugs that block RyR2 specifically for the treatment of cardiac arrhythmias, some of which will be discussed below.
JTV519 and Related Compounds
JTV519 (also referred to as K201) is a 1,4-benzothiazepine derivative, that in addition to being a non-specific multiple channel blocker, also reduces diastolic SR Ca2+ leak by stabilizing RyR2 (Fig. 17.4) [128, 129]. Although, JTV519 has been shown to reduce diastolic SR Ca2+ leak, the exact mechanisms remain contentious [12, 130]. As per the current school of thought, JTV519 stabilizes the closed state of RyR2 by increasing its affinity for FKBP12.6 [12]. This is supported by studies showing that HF and CPVT are characterized by diastolic Ca2+ leak via RyR2 and depletion of FKBP12.6 and vice versa FKBP12.6 deficiency leads to Ca2+ leak and ventricular arrhythmias indicating the importance of FKBP12.6 in arrhythmogenesis [5, 10, 38]. JTV519 has been shown to inhibit both exercise and epinephrine-induced arrhythmias only in mice that have lower FKBP12.6 levels but not in mice that are completely deficient in FKBP12.6 providing indirect evidence that the effect of JTV519 on RyR2 requires rebinding of FKBP12.6 [12]. This is supported by another study that showed that JTV519 prevents pacing induced HF and restores the stoichiometry of FKBP12.6-RyR2 to normal levels [10]. On the other hand, few studies have shown that FKBP12.6 is not required for an effect of JTV519 on RyR2 [130, 131] and that the effect of JTV519 has been attributed to reversal of domain unzipping to produce a zipped state which reduces SR-mediated Ca2+ leak in failing hearts [103]. Still, even in CPVT animal models that do not have apparent dissociation of FKBP12.6 from RyR2, JTV519 reduces ventricular arrhythmias once challenged with increased Ca2+ load [14, 15]. Thus, JTV519 remains an important investigational drug that has a potential to prevent and/or treat arrhythmias with the added advantage of improvement in cardiac function if used in patients with HF induced arrhythmias [10, 13]. However, its off-target effects on other ion channels, including IKr can lead to prolongation of QTc interval that requires further studies to exclude its proarrhythmic potential [132].
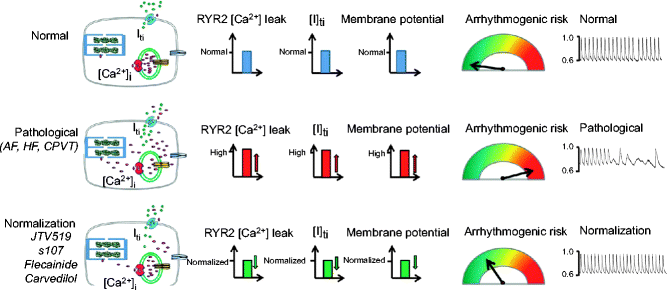

Figure 17–4.
Blockers and stabilizers of ryanodine receptors type 2 (RyR2) prevent arrhythmia. Under normal conditions, RyR2 rarely opens in diastole. Spontaneous opening leads to leaky RyR2 in various pathophysiological situations, generating aberrant Ca2+ sparks and Ca2+ waves that activate inward depolarizing Iti currents via Na+/Ca2+ Exchanger (NCX), that in turn generate delayed after-depolarizations (DADs) and arrhythmia. Compounds that block or stabilize RyR2 prevent Ca2+ leakage and arrhythmogenic risk (Modified from Thiereau et al. [127] with kind permission from Elsevier)
S107 is a derivative of JTV519 that was developed because of its specificity for RyR2 and its favorable drug properties, including drug stability and oral bioavailability [133]. Like JTV519, S107 has been shown to increase the binding of FKBP12.6 to RyR2 and reduce the diastolic SR Ca2+ leak and ventricular arrhythmias in an animal model of CPVT (RyR2-R2474S knock-in mice) [90]. Duchenne muscular dystrophy is also characterized by enhanced diastolic SR Ca2+ leak and ventricular arrhythmias that can be reversed by S107 in animal model [16]. Although S107 seems to be a better drug than JTV519 [133], its applicability might be limited to arrhythmias that are caused by diastolic SR Ca2+ leak.
Flecainide
Flecainide is a Class Ic anti-arrhythmic agent that is approved by FDA for the treatment of ventricular and supraventricular arrhythmias [134–136]. Its predominant mechanism of action is inhibition of fast Na+ channels resulting in slowing of atrial and ventricular conduction [134]. However, recent work has shown that flecainide also acts as an open state RyR2 blocker [17, 137]. Studies have shown that flecainide reduces diastolic SR Ca2+ leak and ventricular arrhythmias in an animal model of CPVT and also in three patients with this disease [17, 18]. Despite the promising results, there are several limitations in the use of this drug. Flecainide causes significant electrical heterogeneity that can both suppress and provoke arrhythmias [138]. The drug also depresses cardiac function and is contraindicated in patients with moderate to severe cardiac failure [139]. Lastly, it suppresses sinus node function and cardiac conduction and is contraindicated in patients with sinus node dysfunction and conduction blocks [134]. The Cardiac Arrhythmia Suppression Trial (CAST) showed that flecainide increases all-cause and arrhythmic deaths in patients with recent myocardial infarction [140]. Despite all the limitations, flecainide provides an exciting opportunity to treat CPVT patients with ventricular arrhythmias due to abnormal SR diastolic Ca2+ leak in the absence of structural heart disease and is currently being evaluated in clinical trials with promising results [141]. In addition, further modification of this drug to increase its specificity of action on RyR2 may eliminate its proarrhythmic properties making it a powerful tool in the treatment of RyR2 leak mediated arrhythmias.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


