Chapter 11 Bronchoscopy
Types of Bronchoscopy and General Instrumentation
Rigid Bronchoscopy
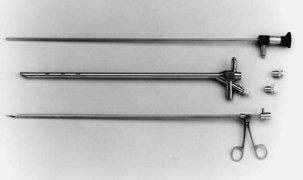
Both rigid and flexible modern systems are equipped with optic capabilities for airway observation alone. With the rigid scope, various types of telescopic rods, equipped with circumferential illumination, permit direct and magnified visualization (Figure 11-1). Specially designed telescopes allow viewing not only directly forward but also at oblique and lateral angles. Various diagnostic and therapeutic instruments can be inserted through the rigid scope while the patient remains ventilated. Rigid bronchoscopy allows a number of therapies such as laser photoresection, endobronchial stents, balloon dilation, electrocautery, argon beam coagulation, and cryotherapy to be performed safely and effectively. Perhaps most important, a rigid scope can be used to “core out” large bulky airway tumors and to dilate central airway strictures and areas of stenosis very effectively and efficiently. In addition, the rigid bronchoscope also can be used for the passage of a flexible scope, which may be necessary for dealing with tortuous airways or distal lesions.
Flexible Bronchoscopy
The flexible scope is used in most bronchoscopic procedures. Although initial flexible bronchoscopes used fiberoptic systems, most such instruments now use a charge-coupled device (CCD) camera at the tip that allows transmission of digital images to a monitor. The main advantages of flexible scopes include their ease of manipulation and greater flexibility, allowing a more complete tracheobronchial tree evaluation than with rigid bronchoscopy, and a less challenging path to expertise, permitting more rapid acquisition of skills (favorable learning curve), for use of these devices (Figure 11-2).
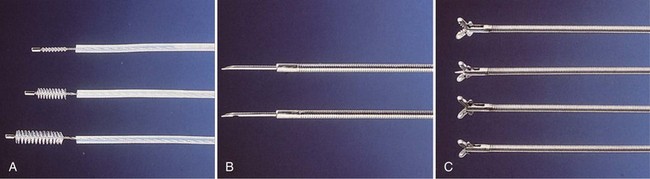
Flexible scopes vary in size, ranging from ultrathin devices allowing for endoscopy in infants and neonates to larger, adult-sized therapeutic scopes. The working channel of the bronchoscope can be used for aspiration of secretions and to accommodate various diagnostic or therapeutic accessories. Four main diagnostic tools have been developed for use during bronchoscopy in order to obtain diagnostic material: bronchoalveolar lavage (BAL), brushings, forceps biopsy, and needle aspiration (Figure 11-3). Since the inception of bronchoscopy more than 100 years ago, these diagnostic modalities have been hampered by limited ability to ensure direct localization of pulmonary nodules, masses, infiltrates, or lymph nodes. However, recent technologic developments in navigational technology and endoscopic ultrasound imaging have improved the ability to localize these lesions, to obtain diagnostic tissue, and to prevent unnecessary surgical intervention. The use of endobronchial ultrasound probes is discussed in Chapter 12.
Patient Preparation and Monitoring during Bronchoscopy
All patients undergoing bronchoscopy should undergo a complete prebronchoscopy evaluation, including a medical history, physical examination, and chest imaging (Box 11-1). Although routine laboratory tests are not required, each evaluation should be individualized on the basis of patients’ underlying conditions and the diagnostic and therapeutic procedures planned.
Box 11-1
Prebronchoscopy Checklist
1. Is there an appropriate indication for bronchoscopy?
2. Has there been a previous bronchoscopy?
3. If the answer to the preceding question is yes, were there any problems or complications?
4. Does the patient (and close relative[s] if patient is unable to communicate) fully understand the goals, risks, and complications of bronchoscopy?
5. Does the patient’s medical history (allergy to medications or topical anesthesia) and present clinical condition pose special problems or predispose to complications?
6. Are all the appropriate tests completed and the results available?
7. Are the premedications appropriate and the dosages correct?
8. Does the patient require special consideration before bronchoscopy (e.g., corticosteroids for asthma, insulin for diabetes mellitus, or prophylaxis against endocarditis) or during bronchoscopy (e.g., supplemental oxygen, extra sedation)?
9. Is the plan for postbronchoscopy care appropriate?
10. Are all the appropriate instruments and personnel available to assist during the procedure and to handle the potential complications?
Modified from Prakash UBS, Cortese DA, Stubbs SE: Technical solutions to common problems in bronchoscopy. In Prakash UBS, editor: Bronchoscopy, New York, Raven, 1994.
Technique
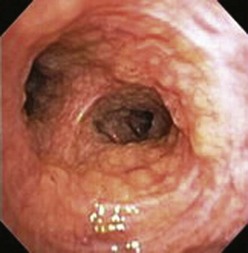
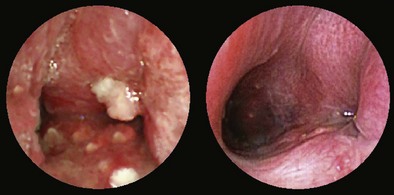
A thorough evaluation of the mucosal surface is an important part of the bronchoscopic examination. The most common abnormality is a change in mucosal coloration, with prominent hypervascular areas seen in patients with chronic bronchitis. The presence of granulation tissue can be due to reaction to a foreign body. Inflammatory mucosal reactions, although not very characteristic, should raise the possibility of mycobacterial infection, nonspecific viral and nonviral infections, and other granulomatous diseases, such as sarcoidosis (Figure 11-4). Mucosal ulcerations are more characteristic of Wegener granulomatosis or malignancy. Loss of the usual mucosal luster and presence of a roughened surface may be early signs of an infiltrative or neoplastic process.
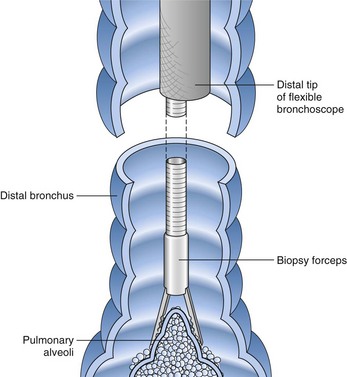
After the bronchoscopic inspection of the airways and surrounding structures has been performed, appropriate samplings should be obtained from the abnormalities identified. Aspirated secretions can be sent for microbiology cultures to determine the offending organism in cases of infection or suspected infection. Endobronchial lesions can be sampled with cytology brushes, biopsy forceps, or needles. Bronchoscopic lung biopsy can be performed for either focal abnormalities or diffuse lung diseases (Figure 11-5). For small or focal lesions, fluoroscopy helps guide peripheral forceps placement and improves the diagnostic yield of biopsies for focal lesions. The use of fluoroscopy also may obviate the need for routine chest radiography after transbronchoscopic lung biopsy. In the case of diffuse lung disease, such as sarcoidosis, use of fluoroscopy has not been demonstrated to improve the diagnostic yield of transbronchial biopsies. Fluoroscopy is useful, however, in providing information regarding the proximity of the forceps to the pleura and in more rapidly establishing the diagnosis of complications (e.g., pneumothorax). Transbronchoscopic needle aspiration (TBNA) and biopsy (TBNB) permit sampling of peribronchial lymph nodes. These transbronchial approaches provide cost-effective diagnostic modalities with less risk and a lower complication rate than with mediastinoscopy (see Chapter 17).
Bronchoalveolar lavage (BAL) is a useful and generally well-tolerated bronchoscopic sampling technique (Figure 11-6). BAL is safe, even in critically ill patients, when biopsy or brushing methods are not recommended because of bleeding risk. Normal saline solution, devoid of any bacteriostatic material, is instilled into distal air spaces through the “wedged” flexible scope and then aspirated through the instrument’s suction channel or from a sterile trap. The fluid collected can be analyzed for gross appearance to detect possible alveolar hemorrhage. The fluid also may be subjected to a variety of tests, depending on the clinical circumstances: microbiologic testing, specific cytologic analysis and cell count, immunologic parameters, presence of various biochemical mediators related to pathologic processes, tissue markers, polymerase chain reaction (PCR) assay, electron microscopy, flow cytometry, and DNA probes. The diagnostic yield of BAL very much depends on specific patient characteristics, the underlying pathologic process, and technical factors.
Indications for Diagnostic Bronchoscopy
Many potential indications have been recognized for both diagnostic and therapeutic bronchoscopy, many of which are listed in Boxes 11-2 and 11-3. The most common reason for bronchoscopy remains the evaluation of a lung mass or nodule. Other major indications include investigation of pulmonary infiltrates, evaluation of opportunistic infections in immunocompromised persons, investigation of hemoptysis, assessment for suspected foreign body, and treatment of airway complications related to neoplasms in the tracheobronchial tree. Some of these indications are discussed next.
Box 11-2
Indications for Diagnostic Bronchoscopy
Box 11-3
Indications for Therapeutic Bronchoscopy
Hemoptysis
Hemoptysis is a common clinical sign and one of the most frequent indications for bronchoscopic evaluation. The most common causes of scant hemoptysis include chronic bronchitis, tuberculosis, and bronchiectasis, whereas massive hemoptysis, usually defined as bleeding greater than 200 mL in a 24-hour period, most often is due to tuberculous cavities, lung cancer, mycetomas, or lung abscess (see Chapter 24). Bronchoscopy can be of help in localizing the site and cause of bleeding. Although the timing of the procedure should be dictated by clinical circumstances, studies have shown that early bronchoscopy (within 48 hours) is more likely to demonstrate active bleeding and allow for the determination of the bleeding site. Chest imaging, with either chest radiograph or computed tomography (CT) scan, can assist in bleeding site localization and, in stable patients without massive hemoptysis, should precede bronchoscopy. In patients with a normal appearance on the chest film, the prevalence of malignancy is approximately 5%, which in most cases is visible by CT scan. The yield of bronchoscopy in patients with normal findings on CT scan is extremely low, and a conservative approach consisting of observation and serial imaging should be considered. Beyond its role as a diagnostic tool, bronchoscopy often can be used to perform various therapeutic procedures in patients experiencing hemoptysis (see further on).
Pulmonary Infections
Bronchoscopy is a useful technique in the diagnosis of pulmonary infections, allowing for the collection of respiratory samples for evaluation with special stains and culture. Respiratory samples can be collected by one or more techniques, including bronchial washing, BAL, protected specimen brushing (PSB), bronchoscopic lung biopsy, and TBNA (Table 11-1).
Table 11-1 Bronchoscopic Techniques and Applications in Respiratory Infections
| Technique | Clinical Applications |
|---|---|
| Bronchoscopy—visualization | Assessment of mucosal, intraluminal, and extraluminal pathology |
| Evaluation of endobronchial tuberculosis, mycoses, viral vesicles (in AIDS) | |
| Evaluation of invasive tracheobronchial aspergillosis, candidiasis, and other conditions | |
| Follow-up evaluation of endobronchial disease (e.g., tuberculosis) | |
| Bronchial washing | Culture for identification of mycobacteria, fungi, and viruses and Pneumocystis smears |
| Bronchoalveolar lavage | Culture for identification of all organisms, especially mycobacteria, fungi, cytomegalovirus and other viruses and Pneumocystis smears |
| Protected specimen brushing | Culture for aerobic and anaerobic bacteria |
| Nonprotected bronchial brushing | Stains and culture for identification of mycobacteria, fungi, Pneumocystis, and viruses |
| Endobronchial biopsy | Mucosal lesions caused by mycobacteria, fungi, protozoa |
| Removal of obstructing lesions responsible for infection (e.g., tumor, foreign body) | |
| Drainage of lung abscess; piecemeal removal of mycetomas (aspergillomas, other fungus balls) | |
| Bronchoscopic needle aspiration | Stains and culture of extrabronchial lymph node specimens for identification of mycobacteria and fungi |
| Drainage of bronchogenic cyst and instillation of sclerosing agent | |
| Bronchoscopic lung biopsy | Stains and culture for identification of all organisms, especially Pneumocystis jiroveci, mycobacteria, and fungi |
| Detection of parasitic lung infections | |
| Rigid or flexible bronchoscopy—therapeutic intervention | Insertion of tracheobronchial prosthesis (stent) to overcome airway obstruction caused by intrinsic stenosis (posttuberculosis or fungal) or extrinsic compression caused by mediastinal fibrosis due to histoplasmosis |
Mycobacterial Infections
In cases in which pulmonary tuberculosis is suspected, the initial diagnostic evaluation should consist of serial examination of sputum for the presence of acid-fast bacilli (AFB) in stained smears. Ideally, induced sputum samples should be obtained. If sputum study results are negative and tuberculosis is still suspected, bronchoscopy with BAL and biopsy should be performed. Both induced sputum collection and bronchoscopy should be performed with appropriate infection control precautions to minimize the risk of nosocomial transmission. A bronchoscopy may cause the patient to produce sputum for several days afterwards; these specimens also should be collected and analyzed, if possible. The utility of bronchoscopy varies widely in the literature, with reported diagnostic yields of 50% to 95%. The yield in patients with miliary tuberculosis, in whom sputum smears frequently are negative, is approximately 70%. Bronchoscopy also is useful in tuberculosis manifesting as an endobronchial lesion or with mediastinal and hilar adenopathy, in which case diagnostic tissue can be obtained with TBNA (Figure 11-7). The yield of diagnostic procedures, including bronchoscopy, can be expected to improve as newer interferon release assays and nucleic acid amplification techniques are incorporated into everyday practice (see Chapter 31).
Bronchogenic Carcinoma
Diagnosis
Peripheral lesions usually are sampled with a combination of bronchial wash, brushes, transbronchial biopsy, and TBNA. The diagnostic yield of bronchoscopy for peripheral lesions depends on a number of factors, including lesion size, the distance of the lesion from the central airways, and the relationship between the lesion and bronchus. The yield of bronchoscopy for lesions smaller than 3 cm varies, ranging from 14% to 50%, compared with a diagnostic yield of 46% to 80% when the lesion is larger than 3 cm. The presence of a bronchus sign on chest CT predicts a much higher yield of bronchoscopy for peripheral lung lesions. In these cases, fluoroscopic guidance should be used to ensure proper positioning of the diagnostic accessory (Figure 11-8).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree