Chapter 4 Bronchoscopic Removal of a Broncholith from the Lateral Wall of the Proximal Bronchus Intermedius
Case Description
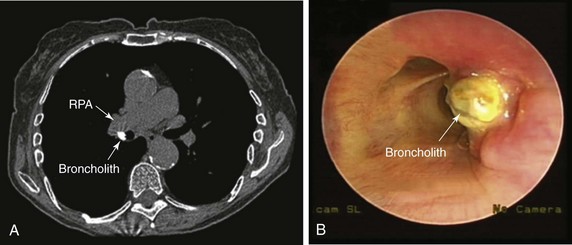
An 82-year-old woman presented with a 2 year history of chronic dry cough. She was treated empirically with antihistamines/decongestants, bronchodilators, inhaled corticosteroids, and proton pump inhibitors for post nasal drip syndrome, asthma, and gastroesophageal reflux disease (GERD), but her cough did not subside. No clinical evidence of aspiration or dysphagia was noted. The cough significantly affected her quality of life because she had developed urinary incontinence and recurrent syncope with severe coughing. She had no other complaints. She lived alone, and at the time of our encounter, she was accompanied by a friend. Physical examination and vital signs were normal. She had diabetes mellitus well controlled with once-daily long-acting insulin and was on no other medications. She had been exposed to tuberculosis when she was young, but she was never diagnosed or treated for active disease. Cardiac workup included dobutamine stress echocardiography, which was normal. Chest radiography and pulmonary function tests were normal. Videofluoroscopic swallow evaluation, 24 hour pH monitoring, and sinus computed tomography (CT) were normal. Noncontrast chest CT performed by her physician showed a calcified lymph node in the right hilum (Figure 4-1). Bronchoscopy revealed a hard, white-yellowish exophytic endobronchial lesion protruding from the lateral wall of the proximal bronchus intermedius (see Figure 4-1).
Case Resolution
Initial Evaluations
Physical Examination, Complementary Tests, and Functional Status Assessment
The diagnosis of broncholithiasis was made after 2 years of unsuccessful treatment for the most common causes of chronic cough. High intrathoracic pressures (up to 300 mm Hg) and velocities (up to 500 miles/hr) make cough an effective means of clearing the airways of excessive secretions and foreign material; however, these physiologic changes can cause a variety of profound physically and psychosocially adverse occurrences that may lead to a significant decrease in health-related quality of life (HRQL).1 The spectrum of complications secondary to chronic cough is broad and includes cardiovascular, gastrointestinal, genitourinary, musculoskeletal, neurologic, ophthalmologic, psychosocial, and respiratory conditions, as well as reduced HRQL; our patient had already experienced loss of consciousness and urinary incontinence. Results of studies show that women with chronic cough are more inclined to seek medical attention than men because their HRQL is significantly more adversely affected, and because they are more likely to experience physical problems such as stress urinary incontinence.2 It is important to exhaust all diagnostic and therapeutic modalities to eliminate cough, if possible, and to not minimize a patient’s complaints.
Our patient underwent a thorough diagnostic evaluation for chronic cough* in accordance with American College of Chest Physicians (ACCP) guidelines.3 She had no evidence of upper airway cough syndrome (aka post nasal drip syndrome), and she did not respond to empirical treatment with antihistamines and decongestants. No clinical or physiologic testing evidence or response to empirical therapy for asthma or GERD was noted. Many clinicians reserve bronchoscopy for patients with chronic cough and suspected lung cancer (based on age and smoking history) even when the chest x-ray is normal. Although bronchoscopy as a primary diagnostic modality is infrequently diagnostic in patients with chronic cough, it may detect laryngeal and tracheobronchial pathology, including broncholithiasis. In our patient, bronchoscopy was performed because the cough persisted after consideration of the most common causes, and because the CT scan suggested possible broncholithiasis, presenting as a high attenuation lesion; the differential diagnosis of such lesions seen on CT can be narrowed by carefully obtaining a patient history and evaluating other CT findings. In cases of broncholithiasis due to erosion by calcified peribronchial lymph nodes, CT usually shows lymph nodes with or without calcification at other locations and parenchymal changes due to previous infection. Our patient, however, had no such findings. Sometimes calcified peribronchial lymph nodes that do not erode into the bronchial lumen may mimic broncholithiasis. In these situations, diagnosis can be confirmed bronchoscopically. In fact, one study showed that bronchoscopy was diagnostic in 7 of 20 (35%) chronic cough patients with unremarkable chest x-ray and without pulmonary or extrapulmonary cancer.4 Two of these seven patients (28%) had broncholithiasis. Although chest CT may be diagnostic, it rarely obviates the need for confirmatory bronchoscopy, especially if therapy is planned.
Although it is not a common cause of cough, studies show that patients with broncholithiasis most frequently present with chronic cough (67% of patients) followed by hemoptysis (38% to 66%), lithoptysis (13% to 19%), fever with sputum production (6% to 15%), dyspnea (15%), wheezing (11% to 15%), and chest pain (4%).5 It is thought that repeated physical impingement of calcified peribronchial lymph nodes on the bronchial wall during respiratory motion is responsible for broncholith formation. When calcified lymph nodes compress or invade the bronchial lumen, changing the bronchial lumen shape, irritating the mucous membrane, and eroding the luminal wall, the previously mentioned clinical manifestations occur, which may lead to recurrent pneumonia or fistulas to the esophagus or the bronchial or even pulmonary artery.6
Our patient likely developed broncholithiasis after a pulmonary fungal infection or tuberculosis, even though the lung parenchyma showed no sequelae of prior lung disease. Some cases may be caused by histoplasmosis or by inflammatory stimuli such as silicosis and foreign bodies.7 Although the most common sequelae of histoplasmosis are asymptomatic pulmonary calcifications and calcified lymph nodes, for which no intervention is warranted, progressive complications such as pulmonary and mediastinal granulomatous disease, fibrosing mediastinitis, and broncholithiasis can occur. One study reported broncholithiasis in 27% (13/49) of patients with histoplasmosis-related complications.8 Our patient had no other radiographic findings suggesting histoplasmosis such as calcified lung nodules or splenic granulomas. Consistent with results from published studies, our patient’s broncholith was on the right side. Sites of predilection for broncholithiasis are on the right side owing to the greater number of pulmonary lymph nodes.9 Some studies report that the bifurcation of the right middle lobe and right lower lobe and the bifurcation of the anterior segment of the left upper lobe and the lingular bronchus were also sites of predilection for broncholithiasis, because the bronchi form an acute angle and the bifurcation has no cartilage rings, making it easy for calcified lymph nodes to penetrate into the bronchial lumen.
When combined, CT scanning and bronchoscopy can clearly determine the type of broncholithiasis: intraluminal (free broncholiths) or penetrating (partially eroding broncholiths). This classification is not just of academic interest in that the type of broncholith identified guides management. Intraluminal broncholiths can lead to distal obstructive inflammation, and bronchial lumina might be obstructed by granulation tissues caused by broncholith-induced long-term airway irritation; these may be extracted by flexible or rigid bronchoscopy.9 Penetrating broncholiths can cause damage to blood vessels and hemoptysis, sometimes even in death. For these patients, although bronchoscopy can be considered in cases of persistent hemoptysis combined with fistulas of trachea, bronchus, or esophagus, or severe secondary pulmonary infection, thoracotomy may be warranted.5,10 On the basis of CT scan and bronchoscopy, our patient was diagnosed as having a penetrating broncholith with none of the already mentioned complications.
Comorbidities
This patient had diabetes mellitus. Interventions provided under general anesthesia can significantly increase risks for perioperative complications. Careful assessment of diabetic patients before surgery is required because of their high risk of coronary heart disease, which may be relatively asymptomatic compared with the nondiabetic population. Diabetes mellitus is also associated with increased risk of perioperative infection and postoperative cardiovascular morbidity and mortality.11,12 Although the risk of surgical wound infection is real should thoracotomy be required, cardiac ischemia becomes unlikely in the presence of a negative dobutamine stress echocardiography. No evidence of other diabetes-associated conditions, such as hypertension, chronic kidney disease, cerebrovascular disease, and autonomic neuropathy, was noted; these conditions can complicate anesthesia and postoperative care.
Support System
The patient’s age and diabetes mellitus status put her at risk for what is known as geriatric syndrome, which comprises functional disabilities, depression, falls, urinary incontinence, malnutrition, and cognitive impairment. Geriatric syndrome leads to frailty, loss of independence, and low quality of life.13 This patient had no immediate family, but she was close to her friend, who was very supportive. In fact, although she was independent in activities of daily living and did not lack decision-making capacity, she did have an advance directive in the form of durable power of attorney for health care, identifying her friend as the surrogate decision maker for health care in case she lost decision-making capacity.
Patient Preferences and Expectations
Clinicians are not always obligated to grant requests for interventions that are clearly ineffective or that violate their conscience.14 This patient was able to clearly express her desire for treatment and wanted her cough to improve so she could live a decent life. Her expectations were considered reasonable, and we decided to honor her request because it was within the standard of care. Her friend was involved in these conversations, and both agreed to proceed with available therapeutic options for broncholithiasis.
Procedural Strategies
Indications
A bronchoscopic procedure could be offered to remove the broncholith (broncholithectomy) and improve her disabling cough. Several treatment modalities have been shown to improve symptoms or manage complications related to broncholithiasis. Treatment ranges from nonoperative management (simple observation) to bronchoscopic broncholithectomy and even thoracotomy for patients in whom severe complications develop. Removal of the broncholith in this patient could prevent the development of hemoptysis, atelectasis, post obstructive pneumonia, bronchiectasis, and even bronchoarterial or bronchoesophageal fistulas.15 Furthermore, removal of the broncholith and its histologic evaluation would exclude alternative diagnoses that could be associated with or mimic broncholithiasis. For instance, primary endobronchial infection with dystrophic calcification (i.e., calcifications of fungus balls within ectatic bronchi), hypertrophied bronchial arteries with intramural protrusion, calcified endobronchial tumors, and tracheobronchial disease with mural calcification (i.e., tracheopathica osteochondroplastica) all can mimic broncholithiasis.16
1. Primary endobronchial actinomycosis could have similar images.17
2. Carcinoid tumors may show calcification. This is more common in central carcinoid tumors (39%) than in the peripheral type (8%).18,19
3. Hamartoma, when endobronchial, may show a central cartilaginous core and can mimic a broncholith.20
4. Tracheobronchial amyloidosis may be localized in the form of a polypoid nodule with calcification, thus mimicking a broncholith.21
5. Aspiration of radiopaque fragments or in situ calcification of foreign bodies may present with radiologic findings of tracheobronchial calcified nodules.16
6. Other less common calcified endobronchial tumors include osteomas, osteosarcomas, chondromas, and chondrosarcomas.
7. The bronchial arteries may become enlarged in various diseases, including acute or chronic pulmonary infection, pulmonary thromboembolism, and chronic obstructive pulmonary disease.22 A hypertrophied bronchial artery may protrude into the bronchial lumen, thus mimicking a broncholith at contrast-enhanced CT.22 Careful analysis of images obtained above and below the abnormality on unenhanced CT sometimes is needed to confirm the vascular nature of the lesion. At bronchoscopy, pulsations of the calcified endobronchial lesion should be carefully sought, before biopsy or removal is considered.
Contraindications
No absolute contraindications to rigid bronchoscopy are known. However, the risk of perioperative cardiac complications should be considered in this elderly patient with a history of diabetes mellitus. Our patient had no clinical signs or symptoms of coronary artery disease and had a normal dobutamine stress echocardiography. Endoscopic procedures (e.g., bronchoscopy) are considered to present low cardiac risk (reported risk of cardiac death or nonfatal myocardial infarction [MI] generally less than 1%), and intrathoracic surgery introduces intermediate risk (reported risk of cardiac death or nonfatal MI generally 1% to 5%).23
Expected Results
The objective of the procedure was to remove the endobronchial component of the calcified lymph node without perforating the airway wall and causing hemorrhage. We intended to leave one piece of broncholith embedded in the bronchial wall intact, if necessary, thus avoiding potential bleeding from the immediately adjacent pulmonary artery. Rigid bronchoscopic intubation was planned using a 12 mm diameter Efer-Dumon ventilating rigid bronchoscope (Efer Broncho, Marseilles, France); this scope allows passage of laser fiber, rigid suction catheter, and forceps, and because of the side-holes at its distal aspect, ventilation to the contralateral left lung would be possible while working in the right bronchial tree. Nd:YAG laser would be available should photocoagulation be needed at the area of insertion in the bronchial wall, coagulation of associated granulation tissue, or bleeding. Lasers (Nd:YAG, pulsed-dye, and holmium-yttrium aluminum garnet [Ho:YAG]) were reported to be useful for fragmenting an eroding broncholith that could not be dislodged with a rigid or a flexible bronchoscope, or for fragmenting a mobile broncholith that was too hard to be broken with the biopsy forceps and too large to be pulled through the upper airway.24–26 Several reports describing use of the laser were limited to removing associated granulation tissue, not the broncholith per se.27 Others used laser to shatter the broncholith when a significant part of the broncholith protruded into the lumen.27 The shattering effect described in the literature can be achieved by applying high laser power (80 to 100 W) to the smallest surface area (high-power density) and very short pulses (0.2 to 0.3 second) interspaced by rest periods (2 to 5 seconds) to avoid overheating of the broncholith and the possible resulting popcorn* effect in adjacent tissues.
Success rates as high as 87% for bronchoscopic removal of broncholiths, without life-threatening complications, have been reported.28,29 The outcome of bronchoscopy depends, however, on the type of broncholithiasis. Several surgical series have reported different outcomes of bronchoscopic broncholithectomy. Among 63 patients studied by Arrigoni et al., broncholiths were removed bronchoscopically from 40 patients (63%) whose bronchoscopies revealed visible broncholiths. The authors concluded that bronchoscopic extraction of a visualized broncholith was “reasonable” as long as irreversible distal bronchial and parenchymal damage had not occurred.30 Based on their successful bronchoscopic removal of intraluminal broncholiths from eight patients without severe bleeding, Cole et al. likewise concluded that bronchoscopic broncholithectomy was a “useful adjunct” and should be thoughtfully attempted before complications of broncholithiasis occur.29 Trastek et al. achieved complete bronchoscopic broncholith removal in 8 of 12 patients (66.7%) who underwent bronchoscopic extraction. Broncholithiasis recurred in three of these eight patients, for which one underwent repeat bronchoscopic broncholithectomy, one required right middle lobectomy, and one refused further intervention and died of massive hemoptysis. Three of four patients who underwent unsuccessful bronchoscopic removal attempts went on to surgery.31 The authors concluded that bronchoscopic broncholithectomy “should probably be reserved for patients who are in poor medical condition.” In an older case series, Faber et al. reported bronchoscopic removal in only 2 of 33 patients studied.32 The authors concluded that bronchoscopic broncholith removal was indicated only if the broncholith was “loose and mobile” and extraction did “not require extensive manipulation.”
In one of the largest published studies, bronchoscopic removal of 71 broncholiths (56% of total identified) was attempted in 48 patients (50.5%) during 61 bronchoscopy sessions. Forty-eight of the broncholiths selected for removal were partially eroding into the tracheobronchial lumen, and 23 were free. Forty-eight percent (23 of 48) of the partially eroding broncholiths were successfully removed bronchoscopically; a greater percentage of broncholiths were removed with the rigid bronchoscope (67%) than with the flexible bronchoscope (30%). All free broncholiths were completely extracted regardless of the type of bronchoscope used. Complications occurred in only two patients (4% of the bronchoscopic removal group), both with partially eroding broncholiths, and consisted of hemorrhage in one patient requiring thoracotomy and acute dyspnea in another patient, caused by a loose broncholith lodged in the trachea.5
Team Experience
Flexible and rigid bronchoscopic extractions of broncholiths are considered safe and effective.5 However, when rigid bronchoscopy is performed, the operator needs to be skilled in gently manipulating the scope inside the airway during the broncholithectomy process to avoid airway wall perforation. Furthermore, because concern for hemorrhage is real in this type of broncholithiasis, the team needs to be ready to respond in case of massive intraoperative hemoptysis. This procedure should not be performed in a facility that does not have the necessary equipment to safely manage a patient’s hemoptysis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree