Diagnosis
BAL features
Alveolar hemorrhage
Macroscopically bloody
Increased intensity from fraction to fraction
Free red blood cells
Hemosiderin-laden macrophages
Fragmented red blood cells in the alveolar macrophages
≥20% siderophages
Alveolar proteinosis
Milky fluid, PAS + acellular corpuscles
Foamy alveolar macrophages
Large amounts of amorphous debris, weak PAS+
Cigarette smoking
Three- to fivefold increase of size of alveolar macrophages
Inclusion bodies (cytoplasmic inclusion of tar products, lipids, lipofuscin)
Eosinophilic pneumonia
Eosinophils >25%
Hypersensitivity pneumonitis
Lymphocytosis >25%
Highest lymphocyte count
CD4/CD8 ratio decreased, but variable too
Infection
Infectious organisms by stains or cultures
Langerhans’ cell histiocytosis
CD1a + or Langerin + Langerhans’ cells >5%
Typical smoker’s changings
Lipoid pneumonia
Oily material
Lipid-laden alveolar macrophages
Malignant infiltrations
Malignant cells
Pneumoconiosis
Dust particles in alveolar macrophages
Birefringent material in alveolar macrophages
Asbestosis
Increased asbestos body count
Sarcoidosis
Lymphocytosis >25%, but milder
Moderate lymphocyte count only
CD4/CD8 ratio >3.5 (in the absence of mixed cellular pattern)
A lymphocytic BAL pattern is commonly seen in granulomatous lung diseases, such as sarcoidosis and hypersensitivity pneumonitis (Fig. 16.1a, b), and in the context of immune reactions of the lung to some drugs. However, the number of lymphocytes and the CD4/CD8 ratio can be variable in sarcoidosis. The majority of sarcoidosis cases display an isolated BAL lymphocytosis, while a raised neutrophil count appears to correlate with more severe disease and need for therapy. In hypersensitivity pneumonitis, not only the number of lymphocytes but also the absolute neutrophil and eosinophil counts may be significantly increased. BAL lymphocytosis >50% generally raises a suspicion for the diagnosis of hypersensitivity pneumonitis, and patients with hypersensitivity pneumonitis may exhibit either decreased, normal, or increased CD4/CD8 ratio. BAL lymphocytosis may be present in methotrexate and amiodarone pneumonitis, as well as in beryllium disease. BAL lymphocytosis appears to be common in the cellular variant of nonspecific interstitial pneumonia (NSIP) too. Finally, a subclinical lymphocytic cellular pattern in BAL has been reported in Wegener’s granulomatosis, Crohn’s disease, and primary biliary cirrhosis.
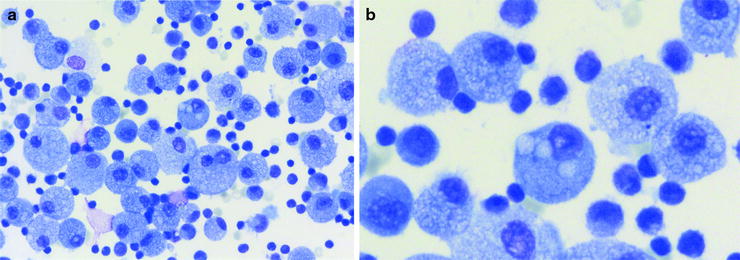

Fig. 16.1
(a) Lymphocytic pattern and foamy cytoplasm of alveolar macrophages with vacuoles in hypersensitivity pneumonitis (HP) (Courtesy of Dr. Henry Budihardjo Welim, Institute of Pathology Hemer). (b) Vacuoles in alveolar macrophages (Courtesy of Dr. Henry Budihardjo Welim, Institute of Pathology Hemer and Dr. Thomas Beyer, Lung Clinic Ballenstedt)
A neutrophilic cellular pattern can be found in idiopathic pulmonary fibrosis (IPF), asbestosis, acute respiratory distress syndrome (ARDS), aspiration pneumonia, subacute hypersensitivity pneumonitis, and cryptogenic organizing pneumonia (COP), as well as in pulmonary infections. An increased neutrophil count may be unspecific but in the appropriate clinical setting is observed in the BAL of 70–90% of patients with IPF. The nonspecific nature of a BAL neutrophilia is illustrated by the difficult diagnostic problem of fibrotic nonspecific interstitial pneumonia (NSIP) too.
An eosinophilic cellular pattern of BAL is in the absence of asthma and parasitic infections highly suggestive of eosinophilic pneumonia. Differential diagnosis may be Churg–Strauss syndrome, allergic pulmonary aspergillosis, drug-induced lung disease, or Langerhans’ cell histiocytosis. The diagnosis of Langerhans’ cell histiocytosis can be made by the presence of more than 5% Langerhans’ cells in BAL, identified by monoclonal antibodies directed against the CD1a antigen or Langerin. Though, it can be an unspecific finding too, as in some cases of IPF, HP, and collagen vascular disease-associated pulmonary fibrosis. If eosinophilia exceeds 25%, eosinophilic pneumonia has to be considered.
Plasma cells are not present in normal BAL. If found, together with foamy macrophages and increased lymphocyte count, HP or drug-induced lung disease has to be suggested. Differential diagnoses are cryptogenic organizing pneumonia and chronic eosinophilic pneumonia. Mast cells appear in the process of lung inflammation and fibrosis. An increased number is sometimes observed in sarcoidosis, associated with advanced or progressive disease.
In addition, if mixed cellular patterns are present, the predominant cellular pattern might offer a hint to the diagnosis, although in these circumstances, invasive procedures such as lung biopsy (bronchoscopic transbronchial or surgical) may be required to make a specific diagnosis.
Specific BAL Findings
In some rare diseases, in the appropriate clinical setting, BAL findings can be diagnostic per se. BAL has a high diagnostic value in these cases (Table 16.2).
Table 16.2
BAL pattern, most important diagnoses, remarks
BAL pattern | Diagnosis | Remarks |
|---|---|---|
Lymphocytic | Hypersensitivity pneumonitis | Highest numbers of lymphocytes |
Highest cell counts | ||
Lymphocytosis >50% | ||
Low CD4/CD8 most common | ||
Foamy macrophages | ||
Plasma cells may be present (antigen exposure) and transient neutrophil count | ||
Sarcoidosis | Mostly isolated moderate lymphocytosis | |
Neutrophils and mast cells may be present | ||
CD4/CD8 ratio >3.5, but high variability | ||
Consider transbronchial lung biopsy | ||
Consider EBUS-TBNA | ||
Nonspecific interstitial pneumonia | Cellular variant | |
Higher lymphocyte count | ||
Lower neutrophil count | ||
Eosinophils may be present | ||
Cryptogenic organizing pneumonia | Lymphocytes dominantly increased | |
Neutrophils, eosinophils, and mast cells increased | ||
Typical symptoms and radiological findings | ||
Drug-induced lung disease | Dominance of CD8+ cells | |
Silicosis | Dust particles in alveolar macrophages | |
Tuberculosis | Staining/cultures for Mycobacteria | |
Radiological appearance | ||
Neutrophilic | Idiopathic pulmonary fibrosis | HRCT findings |
Moderate increased neutrophil count (10–30%) in 70–90% of patients | ||
Eosinophils slightly increased (in 40–60% of patients) | ||
Neutrophils >2× eosinophils | ||
Collagen vascular disease | Dominantly increased neutrophils | |
Asbestosis | Asbestos bodies (negative in 10–15%) | |
Bacterial infection | Bacteria on staining and cultures | |
Eosinophilic | Eosinophilic pneumonia | Eosinophils >25% (up to 90%) |
Eosinophils > neutrophils | ||
Plasma cells may be present | ||
Radiological criteria on HRCT | ||
Churg–Strauss syndrome | Moderate eosinophilia | |
Allergic bronchopulmonary aspergillosis | Staining for Aspergillus+ | |
Criteria for ABPA | ||
Drug-induced lung disease | Very variable | |
Mixed | Predominant pattern may lead to diagnosis |
In pulmonary alveolar proteinosis, the BAL fluid looks milky or turbid. Under bronchoscopy, this may cast suspicion on the specific disease. Under light microscopy, the characteristic acellular oval bodies (surfactant-derived lipoproteins) are basophilic on May-Grünwald–Giemsa staining and positive with PAS staining. The background is filled by large amounts of amorphous debris showing weak PAS staining and few foamy macrophages (Fig. 16.2).
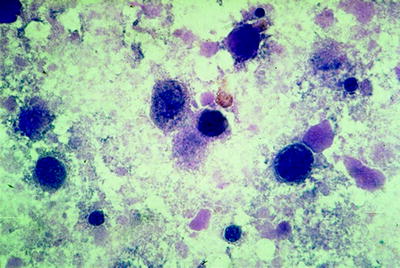

Fig. 16.2
PAS-positive acellular bodies, background filled by debris in pulmonary alveolar proteinosis (Courtesy of Dr. Thomas Beyer, Lung Clinic Ballenstedt)
The combination of grossly milky BAL fluid, PAS-positive acellular oval bodies, and foamy macrophages under light microscopy is virtually pathognomonic of the disease and obviates the need for transbronchial or surgical lung biopsy.
Pulmonary Langerhans’ cell histiocytosis is strongly associated with cigarette smoking, and the BAL differential cytology shows a typical smoker constellation with increased total cell counts and macrophages with smoker’s inclusions. The specific finding is an increase in Langerhans’ cells to >5% of the total BAL cell count (Fig. 16.3). The sensitivity is low because in late cases of the disease the number of Langerhans’ cells decreases in the tissue. Low proportions of Langerhans’ cells in the range of 2–4% can be seen in other conditions, such as in healthy smokers, respiratory bronchiolitis/interstitial lung disease (RB/ILD), other ILDs, and bronchoalveolar carcinoma. Staining by monoclonal antibodies for CD1a or Langerin enables identification of Langerhans’ cells in BAL (Fig. 16.4). The reaction with the polyclonal antibody S100 is less specific. In cases with characteristic BAL findings, electronic microscopy is not needed.
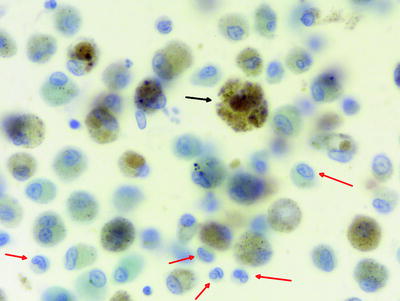
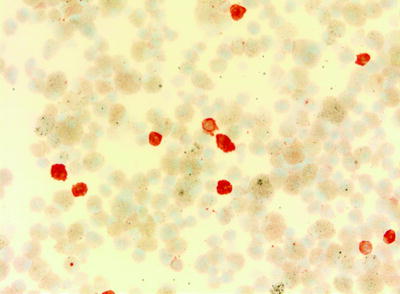

Fig. 16.3
Alveolar macrophages (black arrows) with smoker’s inclusions and Langerhans’ cells in pulmonary Langerhans’ cell histiocytosis (red arrows). Note the notches of the nuclei of Langerhans’ cells (Courtesy of Dr. Henry Budihardjo Welim, Institute of Pathology Hemer)

Fig. 16.4
Staining by monoclonal antibodies for CD1a identifies Langerhans’ cells (Courtesy of Dr. Henry Budihardjo Welim, Institute of Pathology Hemer)
Multiple causes may lead to diffuse alveolar hemorrhage (DAH). It is a clinical syndrome characterized by severe hemoptysis because of bleeding into the alveolar space. BAL analysis may help to diagnose alveolar hemorrhage syndromes, including Goodpasture’s syndrome, Wegener’s granulomatosis, systemic lupus erythematosus and other vasculitides, idiopathic pulmonary hemosiderosis, pulmonary capillaritis, and collagen vascular disease. The characteristic findings in BAL are numerous hemosiderin-laden macrophages. If coagulopathy is excluded, BAL is pivotal in excluding or confirming diffuse alveolar hemorrhage in patients with unexplained pulmonary infiltrates. In extensive diffuse alveolar hemorrhage, hemoptysis is often minimal or absent, and HRCT findings are nonspecific.
Fresh bleeding leads to free red blood cells in the BAL fluid. Fragments of ingested red blood cells within the cytoplasm of macrophages are pathognomonic. The color of the BAL fluid is bloody or something between pink and brown, depending on the interval and intensity to bleeding. The recovered fluid stains more intensely from fraction to fraction, which is characteristic for alveolar hemorrhage. It can be distinguished from aspirated blood from the bronchi by the fact that then the first fraction is the bloodiest one.
To assess the severity of bleeding, the percentage of siderophages can be counted. This is more practical than the time-consuming application of the Golde score, which also takes into account the intensity of staining of each macrophage. It has been shown that a percentage of siderophages ≥20% is sufficient for the diagnosis of DAH. Hemosiderin-laden macrophages do not appear earlier than 48 h after bleeding. Thus, very early bleeding shows only numerous red blood cells.
It is important to highlight that many syndromes belong to this group of disorders; therefore, other clinical and laboratory findings must be considered to establish the cause of bleeding. In the clinical setting, chronic left heart failure with pulmonary congestion is one of the most frequent underlying conditions for the finding of DAH in BAL fluid examination.
BAL is not as sensitive for solid tumors as biopsy or other cytology techniques. But diffuse malignant infiltrates can be reliably diagnosed in 60–90% of cases. Malignancies like primary bronchoalveolar carcinoma or lymphangitis carcinomatosis due to adenocarcinoma have the highest yield in BAL. BAL can also provide diagnostic cytological material in hematological malignancies of the lung, including lymphoma, leukemia, and others.
Different pneumoconioses lead to changes which can be detected through the use of BAL. Dust particles in alveolar macrophages can confirm exposure, but no close correlation exists between the extent of disease and the quantity of inhaled dust. Dust particles and birefringent material within the alveolar macrophages or elevated asbestos body counts suggest occupational exposure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


