Respiratory
Chronic
COPD/asthma
Sepsis
Bronchiectasis
Cystic fibrosis
Cancer
Lung cancer
Mesothelioma
Intrathoracic metastases
Fibrosis
Respiratory muscle weakness due to cachexia
Neuromuscular
Motor neurone disease
Muscular dystrophies
Skeletal
Chest wall abnormalities
Acute
Pneumonia
Emphysema
Pneumothorax
Pulmonary vascular
Pulmonary thromboembolism: acute and chronic (recurrent)
Pulmonary hypertension: primary and secondary
Cardiac
Chronic
Heart failure (right, left, or congestive)
Arrhythmias (particularly atrial fibrillation)
Acute
Coronary events
Psychological
Anxiety, depression, hyperventilation
Anemia
Cachexia
Mechanisms of Dyspnea in Chronic Lung Disease
Hyperinflation
In diseases that cause airway obstruction, for example, asthma and chronic obstructive pulmonary disease (COPD), in an attempt to obviate the degree of airway narrowing, patients tend to breathe at a higher lung volume; closer to total lung capacity (TLC) than usual (Fig.2.1). In this way lung tissue is stretched and the airways that are embedded within it experience a greater retractile force and are, to some extent, widened. In that sense the strategy works. This, of course, is not a deliberate or even conscious change; patients are unaware that they are breathing at a higher lung volume. There are a number of other consequences to this adaptation which can give rise to specific symptoms. As can be seen, breathing at a higher lung volume implies that the inspiratory capacity is diminished. Therefore, if a patient attempts to take a deep breath in, they will find they reach their limit (TLC) sooner than expected. Unaware that their inspiratory maneuver began at a high lung volume and unaware that they are already at TLC, this limitation to further inspiration will be perceived as an “inability to get a full breath in.”
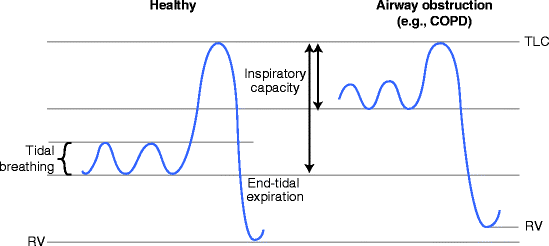

Fig. 2.1
Graph of lung volumes against time in a healthy subject and a patient with airway obstruction (e.g., COPD). In the context of COPD, tidal breathing occurs at a higher lung volume; this helps to support the airway open but has a number of other consequences (see text). Note the inspiratory capacity (the gap between the lung volume at the end of a tidal expiration and the total lung capacity) is reduced in airway obstruction.TLCtotal lung capacity,RVresidual volume
In healthy subjects at the end of a normal tidal expiration, the intrinsic tendency for the lungs to contract is just counterbalanced by the natural tendency for the chest wall to “spring outward.” No muscular effort (no work) is required to hold this “neutral” position. Breathing close to this lung volume is therefore quite effort efficient, like gently stretching and releasing a spring that has no baseline tension. As lung volume increases, lung tissue is stretched, and like the spring it tends to resist further stretch. In fact, unlike the perfect spring of Hooke’s law, the greater the stretch, the greater the force that is required to further expand the lung. When, as in airway obstruction, tidal breathing occurs at a higher lung volume, the muscular effort needed simply to move the chest wall (the work of breathing) is hugely increased. Like attempting to stretch and relax a spring that is already under considerable tension. For a normal degree of ventilation, much greater effort is needed. This “mismatch” contributes to the sensation of breathlessness and is another price paid for airway dilatation achieved by hyperinflation.
To gain some appreciation yourself of how important a factor this is in the perception of breathlessness try it! From a normal lung volume, take a breath in, about half way to full capacity. Then spend just a minute trying to breathe normally, at this hyper-inflated position. A minute will seem like a long time. Remember, those with airway obstruction are breathing at this high lung volume all the time.
Hypoxia
The link between hypoxia and breathlessness is weak. To the lay person, the reason we breathe is to take in oxygen. Whilst this is of course an important imperative, the first priority of the respiratory system is to maintain a normal pH. It is not possible to survive for long with a pH outside of the normal range. Control is achieved by adjustments in pCO2(pH and pCO2are intimately linked). So, if for example a metabolic alkalosis were to arise (e.g., after vomiting), to correct the high pH, ventilation would be reduced and CO2would accumulate. By reducing ventilation however pO2would also fall. Fortunately (within limits) this has no detrimental effect. The level of oxygenation we normally maintain (pO211–14 kPa) is far above what is required to sustain life, even in the long term. A modest fall in oxygenation would not normally be perceived by the individual as breathlessness and it would not, per se, drive up ventilation. Only at a much lower level (pO2around 8 kPa) does the center in the brain stem responsible for protecting us from hypoxia wake up and start to take action.
Prevalence of Breathlessness in Advanced Respiratory Diseases
Studies of symptom prevalence in advanced diseases show wide variation. This variation is dependent on the symptom, the disease, and the stage of the disease. A systematic review [3] reported the prevalence of breathlessness in cancer studies at 10–70 % (10,029 patients) and in advanced COPD studies at 90–95 % (372 patients). In one cancer study [4], the incidence of dyspnea was 84 % in lung cancer and the next most frequent was 58 % in lymphoma. In this study, they also assessed severity of breathlessness by a dyspnea score. The most severe dyspnea was observed in lung cancer.
Idiopathic pulmonary fibrosis (IPF) is characterized by dyspnea. Kozu et al. [5] studied 65 stable IPF patients and correlated their Medical Research Council (MRC) Dyspnoea Grade [6] with exercise capacity and lung function. The percentage of subjects in MRC Grades 2, 3, 4, and 5 was 25, 26, 26, and 23 %, respectively. The MRC Grade correlated positively with 6 minute walking distance (6MWD) and gas transfer (DLCO).
Assessment of the Breathless Patient
Apart from understanding the underlying cause for dyspnea, it is also important to assess the impact that the symptom has on the patient. This applies both to the individual practitioner managing a particular patient and also to the researcher investigating new interventions. Such assessments may include qualitative descriptors of the experience, quantitative measures of the symptom and its influence on quality of life and functional assessments such as exercise tests. It is obvious that the choice of application of these will depend on the status of the patient and his/her wishes. An extensive literature on assessment tools already exists [7,8].
Attempts to identify the underlying cause of breathlessness by studying the words or phrases used to describe the experience have largely been unsuccessful [9]. Although there do seem to be clusters of descriptors in specific conditions, such as the inspiratory effort needed in COPD, their usefulness in the assessment of the breathless patient is limited.
Quantification of the symptom is better evidenced and more relevant [7]. The Medical Research Council Respiratory Symptoms Questionnaire (MRCD) [6] is well validated and simple to apply, and has been used for other respiratory conditions too [5]. Another simple scoring scale of severity is the Numerical Rating Scale (NRS) [10]. This is a scale from 0 to 10 (no breathlessness up to the worst possible).
Apart from the actual symptom, there are a number of other concomitant symptoms that the breathlessness patient may experience at the same time, such as fatigue, mood changes, and loss of control. These are captured in the Chronic Respiratory Disease Questionnaire (CRQ) [11]. In the CRQ, the patient chooses those situations most important in terms of impact of dyspnea on his/her life. The questionnaire may then be self or healthcare professional administered. Four domains are identified: dyspnea, fatigue, emotional, and mastery.
In the palliative context, it is rarely necessary to demonstrate or measure functional impairment objectively. If it is felt necessary, then exertional dyspnea can be simply assessed with the 6 Minute Walking Test (6MWT) [12]. Breathlessness at rest can be quantified by counting numbers [13]. This simple test involves the patient reading out aloud randomly generated two digit numbers over a 1 min period. From this the observer measures the total number of numbers and also the number of breaths taken. Thus the number of numbers per breath is calculated.
Management of the Breathless Patient
General Principles
The first stage after an assessment of the underlying cause of dyspnea will be to offer disease-modifying treatment in an attempt to reverse the process. Mostly this will be drug treatment, but, particularly in cancer-related dyspnea, radiotherapy or physical interventions may also be employed. These will be discussed in the lung cancer or mesothelioma chapters.
Purely symptomatic strategies for breathlessness management include:
1.
Pharmacological management
2.
Non-pharmacological management
3.
Oxygen
Pharmacological Management of Dyspnea
Of the drugs that have been tried for the symptomatic relief of breathlessness, only benzodiazepines and opioids are widely used. Other drugs such as nebulized furosemide and antidepressants are unproven.
Opioids
The largest evidence base for drug treatment of dyspnea exists for opioids. A systematic review [14] described earlier studies. The conclusions were that there was evidence to support the use of oral and parenteral opioids, but not for the nebulized route. Most of the evidence came from studies in COPD patients, with less evidence for cancer and interstitial lung disease. Two more recent studies [15,16] have explored the use of sustained release morphine and to define a starting dose. In the more recent study [16], it was reported that 10 mg sustained release oral morphine gave a beneficial response rate of 62 % which was not improved by dose incrementation. There were no episodes of respiratory depression in the 83 COPD patients involved in the study.
There are no studies looking at dosing of opioids for dyspnea in patients already taking them for pain.
Suggested Dosing Schedules for Opioids for Breathlessness
In opioid naïve patients, the options include a weak opioid such as codeine phosphate 15 mg 6 hourly as necessary, immediate release oral morphine suspension 1–2.5 mg 6 hourly as necessary, or modified release morphine, 10 mg once daily. Dose titration can be continued as for pain. The usual morphine side effects (constipation, initial drowsiness, and nausea) may well be encountered. Parenteral opioids are usually reserved for the end of life when the oral route is not appropriate. If the dose is started low and escalation appropriate, then respiratory depression should not be a problem in stable COPD, but intercurrent infection or other causes of exacerbations may interfere with this.
In patients already on morphine for pain, it is conventional to use short acting morphine at the appropriate rescue dose for dyspnea as well. Towards the end of life, the equivalent subcutaneous dose is used.
Benzodiazepines
The use of benzodiazepines for the palliation of dyspnea is widespread. A Cochrane Review [17] describes the 7 studies involving 200 patients with COPD and advanced cancer. They performed a meta-analysis of 6 studies and concluded that there was no evidence for the beneficial effect of these drugs for breathlessness in these conditions. They noted that whilst drowsiness occurred, it was less than with morphine. The drugs used were alprazolam (2 studies), diazepam (25 mg daily – 1 study), midazolam (8 and 20 mg daily versus morphine – 2 studies), lorazepam 1 mg daily (1 study), and chlorazepate 7.5 mg daily (1 study). They concluded: “These results justify considering benzodiazepines as a second or third-line treatment within an individual therapeutic trial, when opioids and non-pharmacological measures have to control breathlessness.”
Suggested dosing schedules are listed below:
1.
Stable COPD, where rest dyspnea is causing distress daily: diazepam 2 mg once daily.
2.
Dyspnea associated with panic, requiring rapid palliation: lorazepam; 500 micrograms sublingually 6 hourly as required.
3.
End of life respiratory palliation: midazolam 5 mg/24 h CSCI titrated upward as necessary.
Non-pharmacological Management of Breathlessness
Introduction
Non-pharmacological strategies are very varied in nature. Some are techniques are taught by the professional to encourage self-management by the patient, for example breathing retraining. Others require direct input from the therapist, such as acupuncture. A third group are those where a piece of equipment provides the therapy, such as handheld fans, which are self-administered, or applied by the practitioner, such as chest wall vibration. The other variable is whether the intervention involves an exercise component. The non-exercise interventions are well evaluated in a Cochrane Review from 2009 [18]. In this review, they are grouped as single (stand alone) or multi-component.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


