Score
Hemoptysis
0
None
1
Less than twice per week
2
Less than once daily but greater than twice per week
3
Daily, bright red blood or clot
4
Decrease of Hg or Hct more than 10%, greater than 150 ml, requiring hospitalization or leading to respiratory distress
Dyspnea
0
None
1
Dyspnea on moderate exertion
2
Dyspnea with normal activity, walking on ground level
3
Dyspnea at rest
4
Requires supplemental oxygen
Cough
0
None
1
Intermittent, no medication necessary
2
Intermittent, nonnarcotic medication
3
Constant or requiring narcotic medication
4
Constant or requiring narcotic medication but without relief
Pneumonia or elevated temperature
0
Normal temperature, no infiltrate, WBC < 10.000
1
Temperature >38.5 and infiltrate, WBC < 10.000
2
Temperature >38.5 and infiltrate and/or WBC > 10.000
3
Lobar consolidation on radiograph
4
Pneumonia or elevated temperature requiring hospitalization
(B)Lesion-Related Factors:
Biopsy-proven malignancy
Accessibility to the catheter placement
The lesion is endobronchially without extrinsic compression
No significant ulceration
No major vascular involvement
In terms of efficacy, clinical improvement has been reported in numerous series. In a study of 270 patients with advanced lung cancer and recurrent endobronchial lesions that underwent EBB treatment, significant symptomatic improvement was achieved in 80% of the patients with a 5-month median duration of palliative period. Symptomatic improvement response rates were as follows: dyspnea 76%, cough 77%, hemoptysis 92%, lung reaeration 73%, and postobstructive pneumonia 82%. It should be noted that gender, age, and histological type were not found to be predictive of brachytherapy success. Additionally, a survival benefit has been observed in patients undergoing EBB. In a study that included 81 patients with lung cancer who underwent chemotherapy and/or EBRT with persistent endobronchial lesion, a significant survival benefit was found in patients who underwent EBB in comparison with patients who did not (13 months versus 5 months).
Short-term efficacy has been documented by histological biopsy after EBB treatment. In a study of 106 patients with endobronchial lung cancer, who were treated previously with surgical resection, EBRT, or were not eligible for any therapy, underwent treatment with EBB. A complete histologic response was achieved in 60% at 3 months follow-up. The 3 and 5-year survivals were 47.4% and 24%, respectively. Factors significantly associated with treatment failure were high tumor volume (i.e., tumor length >2 cm), bronchial obstruction (i.e., more than >25% of the lumen), tumor visibility on CT scan, and previous endoscopic treatment.
Patients with central malignant lesions showed significant improvement of these symptoms in comparison to patients with peripheral malignant lesions.
EBB has been also used to treat roentgenographically occult endobronchial malignancies. Nineteen patients with limited endobronchial non-small cell lung carcinomas measuring less than 1 cm in diameter and not visible on a CT scan of the chest underwent EBB due to lack of other treatment options. Tumor control over a 2-month period was 83%, and over 1 and 2-year periods was 75% and 58%, respectively. Finally, complete cancer resolution was achieved in few case reports. Two patients with T1N0 squamous cell lung cancer and poor lung function to undergo surgical resection received EBB. A complete cancer resolution at 25 and 54-month follow-up was achieved.
Combination Therapy of EBRT and EBB
In general, external beam radiation therapy (EBRT) alone provides superior and more sustained palliation to patients than EBB alone, with fewer re-treatment sessions, as well as a modest gain in survival time. In patients with advanced inoperable lung cancer, combination therapy with EBRT and EBB has resulted in longer survival durations. However, these durations were not statistically significant in relationship to EBRT alone. This observation was noticed especially in squamous cell carcinoma. Respiratory symptoms (i.e., dyspnea, hemoptysis, cough, and chest pain) were not different between the two groups. Yet patients with atelectasis secondary to endobronchial lesion responded well to the combination therapy. Patients with roentgenographically occult lung cancer treated with combined EBRT and EBB achieved both a high remission rate and low recurrence.
EBB with Other Bronchoscopic Interventional Therapies
Combining EBB with other treatment modalities such as electrocautery, cryotherapy, and Nd-YAG laser therapy to treat inoperable or advanced lung cancers achieves a larger degree of local control of the endobronchial malignant lesions.
EBB and Photodynamic Therapy (PDT)
Brachytherapy can be combined with photodynamic therapy in patients who have nonoperable endobronchial malignancy, malignancy relapse, or tumor residual after PDT. In a series, 32 patients with non-small cell bronchogenic carcinoma and bulky endobronchial tumors were treated using a combination of PDT and brachytherapy. At a mean follow-up of 24 months, 26 patients were free of residual tumor and local recurrence.
EBB and Endobronchial Ultrasound Bronchoscopy (EBUS)
EBUS has been used for EBB treatment planning. It helps in evaluating the extension of the targeted endobronchial lesion, vascular involvement, or lymph-node metastasis, which escaped other images.
Techniques
Initial Evaluation
A flexible bronchoscopy should be performed in the beginning to evaluate the location and the character of the endobronchial lesion, such as the anatomic site and the location of the lesion in reference to a fixed landmark (carina), the length of the lesion, and the degree of the airway obstruction. Speiser and his colleagues proposed scoring criteria for the degree and location of the airway obstruction as displayed in Table 36.2. This information should be recorded for dose prescription purposes.
Table 36.2
Speiser’s obstruction definitions and scoring criteria
Trachea | >50% = 10 | <50% = 6 | <10% = 2 |
Main bronchus | >50% = 6 | <50% = 3 | <10% = 1 |
Lobar bronchus | >50% = 2 | <50% = 1 | |
Atelectasis | 2 per lobe | ||
Pneumonia | 2 per lobe | ||
An adequate passage should be visible through the endobronchial lesion to pass the catheter. If the lesion is occluding the lumen, a passage should be created first by using mechanical debridement, laser, or electrocautery. This procedure should be done in a separate session prior to the brachytherapy treatment.
In patients with early stage central lung malignancy, an adequate assessment with EBUS prior to EBB should be conducted for adequate staging. Lesions with cartilage invasion should be treated as stage IA and should undergo surgical evaluation. Lesions without cartilage involvement will be appropriate for any form of local therapy such as EBB, laser, or electrocautery therapy or mechanical removal.
Catheter Placement
A flexible polyethylene catheter is usually inserted into the airway by using a flexible bronchoscopy with adequate channel size for the catheter delivery. A transnasal approach is used when performing the bronchoscopy (Fig. 36.1). The majority of the cases can be done under conscious sedation, but general anesthesia may be needed in special circumstances. The distal end of the catheter is placed at the site of the endobronchial lesion, and it should be about 2 cm beyond the distal end of the lesion (Figs. 36.2 and 36.3). In the alternative, the catheter may also be placed by wedging the tip of the catheter into a segmental bronchus. This technique helps in minimizing any catheter displacement. It is important to note that the catheter should not be forced when resistance is felt. The bronchoscopy and the guidewire should be removed gently and the catheter marked at the site of its exit from the nostril and secured into position with adhesive tape. The position is verified with fluoroscopy and CXR. A second and third catheter may be placed in similar fashion to more fully surround the tumor.
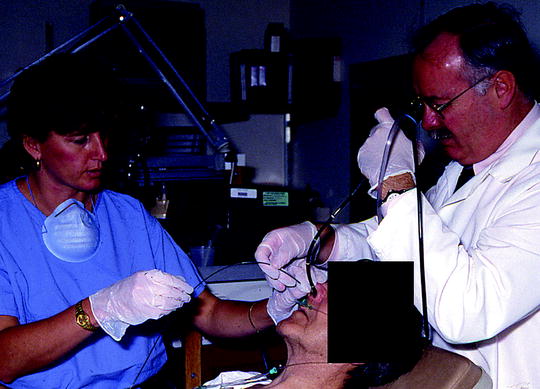
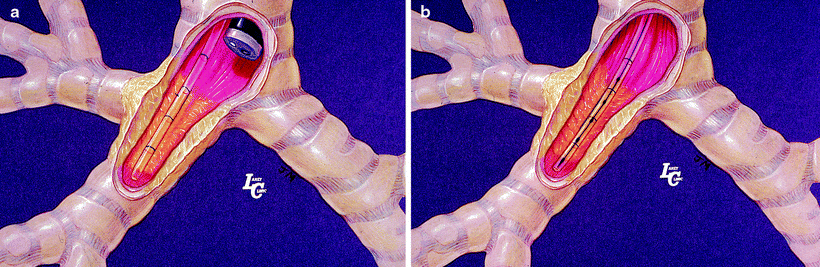
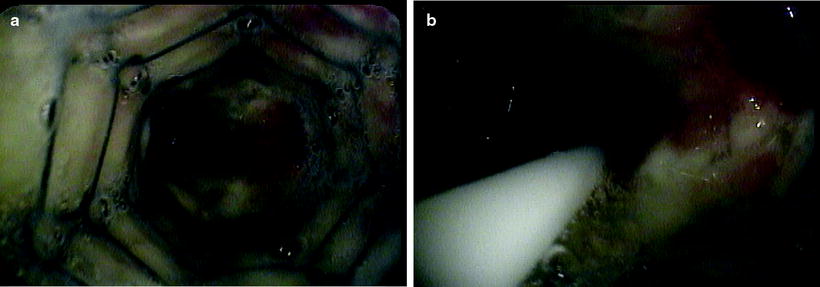

Fig. 36.1
Endobronchial brachytherapy catheter is being adjusted under the bronchoscopic guidance after the initial insertion (Courtesy of Lahey Clinic, Burlington, MA)

Fig. 36.2
(a) Verifying the brachytherapy catheter position in the airway next to the endobronchial lesion with bronchoscopy. (b) The brachytherapy catheter after loading the radioactive source (seeds) (Courtesy of Lahey Clinic, Burlington, MA)

Fig. 36.3
(a) Malignant endobronchial stenosis at the distal end of the left main stem where the metallic stent was placed. (b) The endobronchial brachytherapy catheter is passed distally for about 2 cm beyond the stenosis (Courtesy of Lahey Clinic, Burlington, MA)
Treatment Planning
Nonradioactive (“dummy”) sources are introduced into the catheter to the level of the bronchial obstruction. Radiographic verification is performed to geographically define the eventual position of the radioactive source. Different prescription points (i.e., total dose and number of fractions) have been used. In practice, prescription depths of 0.5–2 cm have been used by numerous investigators. The American Brachytherapy Society suggested two methods:
1.
Prescribing the radiation dose to a fixed distance from the center of the catheter, which is typically 10 mm. This method is used when treating a lesion located in the trachea or major airways.
2.
Prescribing the radiation dose at various distances from the center of the catheter(s), depending on the diameter of the airway. This method is used when treating lesions in smaller airways. It usually ranges between 5 and 10 mm, depending on the bronchus.
The treatment margin should include 1–2 cm at the proximal and distal end of the endobronchial lesion. With advanced technologies, three-dimensional brachytherapy treatment planning may provide more effective and accurate targeting of the tissue.
Treatment Session
The patient should be taken to the Department of Radiation Oncology for manual or remote loading of the radioactive source. Once the catheter is connected to the treatment unit, a calculated dose is delivered. After completing the treatment session, the catheter is disconnected from the treatment unit and removed (Fig. 36.4). Then, the patient may be discharged home with specific instructions to contact the treating physician if acute dyspnea, cough, or hemoptysis occurs. The American Brachytherapy Society suggests grading the bronchoscopic response to the treatment as excellent, good, fair, and poor.
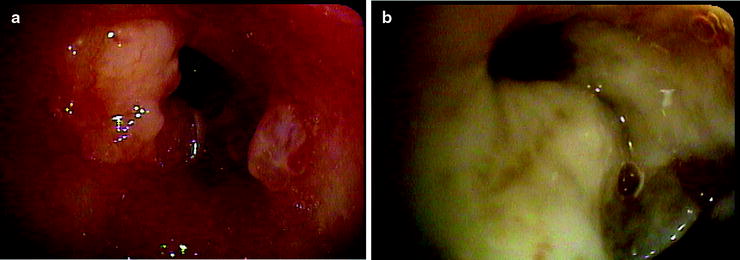

Fig. 36.4
(a) Malignant endobronchial lesion obstructing the left upper lobe. (b) Postbrachytherapy treatment with the pus draining from the postobstructing pneumonia in the same lobe after the resolution of the obstructing lesion (Courtesy of Lahey Clinic, Burlington, MA)
Indications for EBB
The indications for endobronchial brachytherapy recommended by the American Brachytherapy Society are listed in Table 36.3.
Table 36.3




The American Brachytherapy Society recommendations for endobronchial brachytherapy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


