Fig. 15.1
Zoledronic acid reduced percentage of patients with each SRE. Phase III trial of patients with bone metastases from NSCLC/OST who received ZOL or placebo every 3 weeks for up to 21 months. ~50 % of patients had NSCLC; ~7 % of patients had SCLC. SRE skeletal-related event, mets metastases, NSCLC non-small cell lung cancer, OST other solid tumours, RT radiotherapy, SCC spinal cord compression, HCM hypercalcaemia of malignancy (Data from Rosen et al. [5])
Zoledronic acid also significantly decreased the annual incidence of SREs, 1.74 per year versus 1.71 per year for placebo, p = 0.012 and significantly delayed the median time to first SRE compared with placebo (236 days versus 155 days respectively, p = 0.009) [5]. A multiple event analysis using a robust Andersen-Gill model was performed for the overall population. This analysis takes into account not only the number of SREs but also the timing between the SREs, thereby providing a sensitive comparison of the ongoing risk of SREs between two treatment groups.
Zoledronic acid reduced the risk of SREs by 31 % versus placebo in the overall trial population (relative risk, RR = 0.693, p = 0.003). Many patients with lung cancer are diagnosed only after the first SRE. However, pre-existing skeletal morbidity does not preclude the benefits of subsequent therapy with zoledronic acid. Indeed, patients who have already experienced an SRE are at especially high risk for subsequent events. In an exploratory analysis of the zoledronic acid phase III trial in patients with NSCLC and other solid tumors, patients with a history of SRE before study entry had a 41 % increased risk of experiencing an on-study SRE compared with patients with no history of prior SRE (p = 0.036) [36]. In patients with a prior SRE, zoledronic acid produced a significant 31 % reduction in the risk of developing an on-study SRE compared with placebo in a robust Andersen-Gill multiple event analysis, p = 0.009, and significantly reduced the skeletal morbidity rate, 1.96 versus 2.81 events per year for placebo, p = 0.030 [36].
Furthermore, zoledronic acid significantly prolonged the median time to first SRE on study by approximately 4 months compared with placebo in this prior-SRE cohort (215 versus 106 days respectively, p = 0.011). Benefits were also seen in the subset of patients who had not experienced a prior SRE, but without a statistical significance because of lack of the statistical power. This study suggests that zoledronic acid is effective and provides benefits even after the onset of SREs.
Tolerability and Safety Profile of Zoledronic Acid
In the NSCLC stratum of the study, zoledronic acid had an overall safety profile comparable with that of placebo.
The most commonly reported adverse events for zoledronic acid and placebo during the trial were bone pain (48 and 58 % respectively), nausea (47 and 32 % respectively) and dyspnea (45 and 30 % respectively) [37]. The 10 % difference in the incidence of bone pain favoring the zoledronic acid group may reflect either effects from the SRE reduction or an analgesic but there were no differences in analgesic consumption between the groups. There was no significantly lower incidence of palliative radiotherapy to bone in the 4 mg zoledronic acid group versus placebo [25]. There were no grade 4 increases in serum creatinine reported in the NSCLC stratum.
Monitoring of renal function and oral health during bisphosphonate therapy is now recommended to avoid uncommon, but potentially serious adverse events [38, 39].
Because all intravenous bisphosphonates are cleared by the kidneys, renal function and hydration status should be determined before each infusion to ensure renal safety. Infrequently, patients with normal renal function may experience dose and infusion-rate dependent effects on renal function. However, patients with impaired renal function are at a greater risk. Therefore, a reduced starting dose of zoledronic acid is recommended for patients with impaired renal function [40].
Osteonecrosis of the jaw (ONJ) has been reported as an uncommon event in patients receiving bisphosphonates and is characterized by exposed bone in the maxillofacial area with no evidence of healing after 6 weeks of appropriate dental care in the absence of metastatic disease or radiation to the jaw [39]. The reports using the data obtained from retrospective analyses and reviews of medical records databases suggest that the frequency of ONJ in patients with malignant bone disease may be between 0.7 and 12.6 % [41–43].
This wide range in ONJ frequency is likely due to variability in preventive dental measures before and during bisphosphonate therapy, variations in the duration of bisphosphonate treatment, and geographic differences. Preventive dental measures and appropriate oral hygiene have been identified that can significantly reduce the incidence of ONJ during bisphosphonate therapy [39, 44–46]. A pilot study in patients with active ONJ lesions found that local application of a medical ozone oil suspension led to complete ONJ resolution [47].
Biochemical Markers of Bone Metabolism
Zoledronic Acid and Biochemical Markers
In a subset of patients with NSCLC or other solid tumors in the placebo group (238 patients) urinary levels of the bone resorption marker N-telopeptide of type I collagen (NTX) and the serum bone formation marker BALP were assessed approximately every 3 months [48]. High NTX levels (≥100 nmol/mmol creatinine) at baseline were associated with an increased risk of first SRE (RR = 1.85, p = 0.076) and bone disease progression (RR = 1.76, p = 0.029) compared with patients with low NTX levels (<100 nmol / mmol creatinine, Fig. 15.2) [48]. Moreover, compared with patients with low NTX levels, patients with high NTX levels had a more than three-fold increased risk of death (RR = 3.03, p < 0.001) and a 5 month reduction in median survival (3.2 versus 8.2 months for patients with low baseline NTX levels) [48]. Patients with high baseline BALP levels (≥146 IU/L) also had statistically significant increases in risk of disease progression (RR = 1.77, p = 0.005) and death (RR = 1.53, p = 0.003) compared with patients with low BALP levels (<146 IU/L) [48].
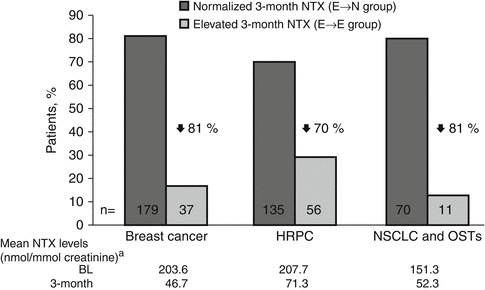

Fig. 15.2
ZOL normalized NTX levels within 3 months in most patients with elevated baseline NTX. NTX N-telopeptide of type I collagen, HRPC hormone-refractory prostate cancer, NSCLC non-small cell lung cancer, OST other solid tumours, BL baseline (Data from Lipton et al. [49])
Zoledronic acid significantly suppresses biochemical markers of bone resorption in patients with bone metastases. In a prospective study measuring levels of bone markers in patients with newly diagnosed bone metastases receiving zoledronic acid every 3–4 weeks (n = 71), zoledronic acid significantly reduced NTX levels at first (55 days) and second (115 days) treatment evaluations (mean reductions of 43 and 45 % respectively), and the levels remained suppressed throughout the study [50]. This reduction correlated with a lower rate of bone disease progression compared with patients whose NTX levels increased (18.8 % versus 66.7 % respectively, p = 0.001) [50].
These results are consistent with data from an exploratory analysis of the zoledronic acid phase III clinical trial database [51] in which zoledronic acid was found to reduce mean urinary NTX levels within 3 months in patients with bone metastases from NSCLC and other solid tumors who had bone marker assessments (n = 204) [49]. Zoledronic acid also significantly reduced the RR of death by 35 % versus placebo (RR = 0.650, p = 0.024) among patients with NSCLC and high baseline NTX levels (NTX ≥ 64 nmol / mmol creatinine, n = 144) [52].
Differences in survival between the zoledronic acid and placebo groups did not reach statistical significance in the normal baseline NTX subset, consistent with the lower risks of SREs and death that have been reported for that subset [48, 52].
This benefit could result from reduced osteolysis, resulting in less release of growth factors from the bone matrix, reduced SRE rate or possibly also from direct and indirect antitumor effects of zoledronic acid i.e. increased apoptosis, synergism with chemotherapy, antiangiogenesis, and stimulation of immune system.
Anticancer Activity of Zoledronic Acid
There is an expanding database of preclinical evidence to suggest that zoledronic acid can inhibit proliferation and induce apoptosis in a broad range of human cancer cell lines [31, 53]. In vitro zoledronic acid inhibits the growth of cell lines derived from human primary tumors including 12 small cell lung cancer cell lines in which it potently inhibited growth with 50 % inhibitory concentration (IC50) values ranging from 13 to 30 mM [54]. Similar effects on cellular viability and proliferation were observed in 16 NSCLC cell lines with IC50 ranging from approximately 2 to 25 mM [55]. Moreover, 10 mM zoledronic acid blocked motility in 3 of these cell lines that were highly motile.
Zoledronic acid also exerts antitumor synergy with chemotherapy agents in the A549 lung cancer cell line [56]. In combination with cisplatin, zoledronic acid 100 mM significantly enhanced cytotoxicity up to 70 % (p = 0.007) compared with cisplatin alone. Zoledronic acid in combination with paclitaxel produced synergistic inhibition of cellular proliferation compared with either agent alone [57]. In a murine lung cancer cell line, zoledronic acid inhibited the growth of these tumors and mice that were treated with zoledronic acid (1 mM/kg/week) survived significantly longer compared with untreated mice (p < 0.05) [58].
Multiple effects may contribute to the antitumor activity of zoledronic acid that has been reported in preclinical models [59]. In addition to direct antitumor effects, nitrogen-containing bisphosphonates appear to have immunomodulatory properties especially with regard to γδ T cells, a subset of T cells that plays a role in immunosurveillance for malignancies. In an in vitro model, zoledronic acid induced maturation and upregulated co-stimulating surface receptor expression (e.g. CD 40, CD 80, CD 83) on peripheral γδ T cells [60]. In addition, bisphosphonates have been shown to activate the cytolytic activity of γδ T cells and therefore, may enhance the antitumor immune response [61].
There are ongoing clinical studies in patients with NSCLC evaluating the efficacy of zoledronic acid both for prevention of bone metastases and for antitumor activity.
Denosumab and Anti-RANKL Activity
Denosumab: Mechanism of Action
Denosumab is a fully human monoclonal antibody that binds to and neutralizes RANKL (receptor activator of nuclear factor kappa-B ligand) thereby inhibiting osteoclast function and preventing generalized bone resorption and local bone destruction.
It is hypothesized that tumor cells in the bone lead to increased expression of RANKL on osteoclasts and their precursors. RANKL is an essential mediator of osteoclast function, formation, and survival [62–64]. Excessive RANKL-induced osteoclast activity results in resorption and local bone destruction with evidence of elevated levels of bone turnover markers, leading to SREs [48, 51].
Denosumab has been studied in two phase II trials of patients with bone metastases in advanced cancer and in one phase II trial with myeloma [65–67]. These studies demonstrated that treatment with denosumab at doses ranging from 30 to 180 mg administered every 4 or 12 weeks was associated with a rapid and sustained suppression of bone turnover markers and delay of SREs similar to that seen with i.v. bisphosphonates.
Denosumab Versus Zoledronic Acid, Phase III Trial
In a randomized, double-blind study (phase III) of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma, 1,779 patients were enrolled onto study, 890 patients analyzed on zoledronic acid, 886 on denosumab [68]. Baseline characteristics were well balanced (Table 15.1). The primary endpoint was time to first on-study SRE comparing denosumab with zoledronic acid for non-inferiority. Secondary efficacy endpoints were to be evaluated only if non-inferiority was demonstrated, and were superiority tests comparing denosumab and zoledronic acid for time to first on-study SRE and time to first and subsequent SRE by multiple event analysis. A subsequent SRE was defined as an event occurring ≥21 days after the previous SRE.
Table 15.1
Baseline characteristics
Characteristic, n (%) or median | Zoledronic acid (N = 890) | Denosumab (N = 886) |
|---|---|---|
Male | 552 (62) | 588 (66) |
Age (years) | 61 | 60 |
Primary tumor type | ||
Non-small cell lung cancer | 345 (39) | 343 (39) |
Multiple myeloma | 93 (10) | 86 (10) |
Other | 452 (51) | 457 (52) |
ECOG performance status of 0 or 1 | 728 (82) | 748 (84) |
Time from first bone metastasis to randomization (months) | 2 | 2 |
Previous SRE | 446 (50) | 440 (50) |
Presence of visceral metastases | 448 (50) | 474 (53) |
The median number of doses was 7 for zoledronic acid and 7 for denosumab with cumulative drug exposure of 651.9 patient years for zoledronic acid and 675.3 patient years for denosumab. Median time on study was approximately 7 months.
Efficacy
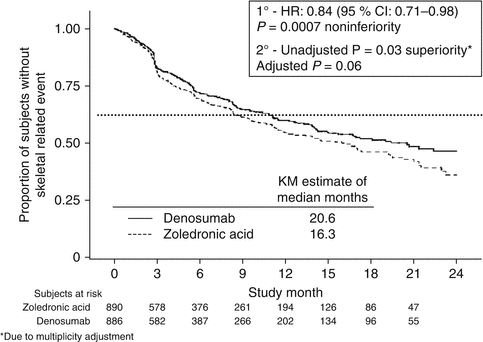
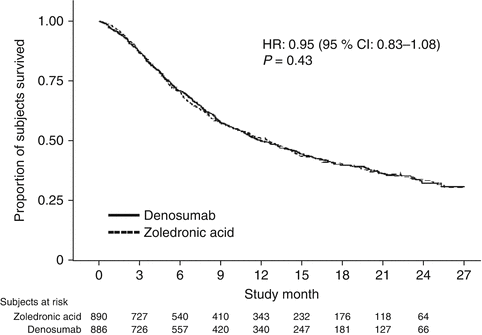
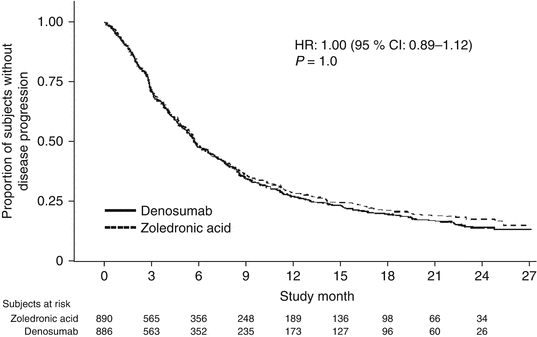
Denosumab was non-inferior to zoledronic acid in delaying time to first on-study SRE (HR = 0.84, p = 0.0007) representing 16 % reduction in hazard (Fig. 15.3). The median time to first on-study SRE was 20.6 months for denosumab and 16.3 months for zoledronic acid. The test for superiority for time to first SRE showed p = 0.06 and therefore did not reach statistical significance. Time to first and subsequent SREs (multiple events) analysis demonstrated a rate ratio of 0.90 for denosumab compared with zoledronic acid, p = 0.14, which was not statistically significant. Overall survival (HR = 0.95, p = 0.43) and disease progression (HR = 1.00, p = 1.0) were similar between treatment groups (Figs. 15.4 and 15.5).
Efficacy by Tumor Stratification
The effect of denosumab on time to first on-study SRE relative to zoledronic acid by tumor stratification factors resulted in an HR = 0.84 for NSCLC, p = 0.20; 1.03 for myeloma, p = 0.89, and 0.79 for other solid tumors, p = 0.04. An ad hoc analysis examining overall survival demonstrated an HR = 0.79 for NSCLC, 2.26 for myeloma, and 1.08 for other solid tumors.
Safety
Patients in both treatment groups experienced similar rates of adverse events (AEs) (Table 15.2). Rates of serious AEs are 13.4 % for zoledronic acid versus 14.6 % for denosumab. New primary malignancy occurred in three patients (0.3 %) receiving zoledronic acid and in five patients (0.6 %) receiving denosumab.
Table 15.2
Adverse events of interest
Event, n (%) | Zoledronic acid (N = 878) | Denosumab (N = 878) |
|---|---|---|
Infectious AEs | 349 (39.7) | 358 (40.8) |
Infectious serious AEs | 118 (13.4) | 128 (14.6) |
Acute phase reaction (first 3 days) | 127 (14.5) | 61 (6.9) |
Potential renal toxicity AEsa | 96 (10.9) | 73 (8.3) |
Renal failure | 25 (2.8) | 20 (2.3) |
Acute renal failure | 16 (1.8) | 11 (1.3) |
Cumulative rates of ONJb | 11 (1.3) | 10 (1.1) |
Year 1 | 5 (0.6) | 4 (0.5) |
Year 2 | 8 (0.9) | 10 (1.1) |
New primary malignancy | 3 (0.3) | 5 (0.6) |
Adverse events of hypocalcemia occurred more frequently with denosumab (10.8 % denosumab, 5.8 % zoledronic acid). In general, the clinical consequences of hypocalcemia were not observed. Centrally determined grade 3 and 4 decreases in albumin-adjusted calcium values were reported in nine patients (1 %) receiving zoledronic acid and 20 patients (2.3 %) receiving denosumab. IV calcium was administered on study to 2.7 % of patients receiving zoledronic acid and 5.7 % receiving denosumab.
Positive adjudicated ONJ occurred with cumulative incidence rates in the zoledronic acid and denosumab groups of 0.6 and 0.5 % at 1 year respectively, 0.9 and 1.1 % at 2 years and 1.3 and 1.1 % at 3 years (p = 1.0).
AEs associated with acute phase reactions within the first 3 days after dose 1 occurred in 14.5 % of patients receiving zoledronic acid versus 6.9 % receiving denosumab. Most frequent reactions were pyrexia, arthralgia, and fatigue. Hundred and fifty two patients (17.3 %) on zoledronic acid required dose adjustments to levels lower than 4 mg and doses were withheld because of elevated serum creatinine in 78 patients (8.9 %). No dose adjustments or dose withholding for renal function were required for denosumab. Despite appropriate adjustments of the zoledronic acid dosing regimen for renal function, there was an evidence of an excess of renal adverse events with zoledronic acid. Denosumab has no limitations with respect to renal impairment as it is a monoclonal antibody and is eliminated by intracellular catabolism in phagocytes, with no evidence of renal effects [70, 71].
Bone Turnover Biomarkers: Denosumab Versus Zoledronic Acid
Patients treated with denosumab experienced a greater suppression of bone turnover markers than with zoledronic acid. Between baseline and study week 13 levels of urinary NTX/Cr decreased by a median of 76 % for denosumab (n = 546) and 65 % for zoledronic acid (n = 543), p < 0.001 and bone-specific alkaline phosphatase decreased by 37 % for denosumab (n = 578) and 29 % for zoledronic acid (n = 581), p < 0.001.
Exploratory Analysis of Overall Survival in Lung Cancer
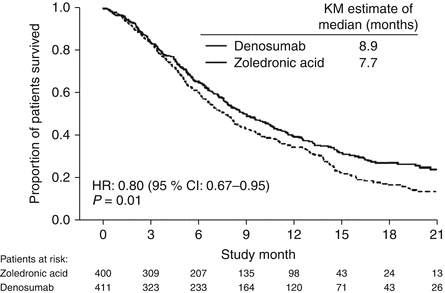
Sub-analysis of 811 patients with any lung cancer showed that denosumab was associated with significantly improved overall median survival compared with zoledronic acid, with a difference of 1.2 months (KM median = 8.9 months versus 7.7 months, HR = 0.80, p = 0.01) (Fig. 15.6) [72]. Denosumab continued to show a significant survival advantage over zoledronic acid when overall survival was adjusted for relevant baseline covariates (age, sex, time from diagnosis of primary cancer to first evidence of metastasis, or the first bone metastasis, visceral metastasis, and ECOG status) and stratified by the randomization stratification factors (previous SRE and systemic anticancer therapy), HR = 0.81, p = 0.01. In patients with visceral metastases (231 in denosumab group and 233 in zoledronic acid group), denosumab was also associated with improved overall median survival with a difference of 1.2 months (KM median = 7.7 months versus 6.4 months, HR = 0.79, p = 0.03). Denosumab was associated with significantly improved survival in patients with NSCLC with a difference of 1.5 months (KM median = 9.5 months versus 8.1 months, HR = 0.78, p = 0.01) (Fig. 15.7).