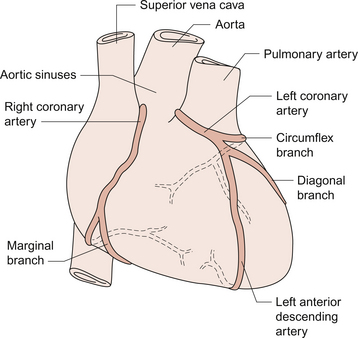
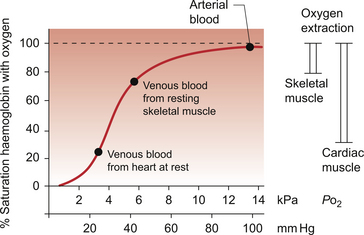
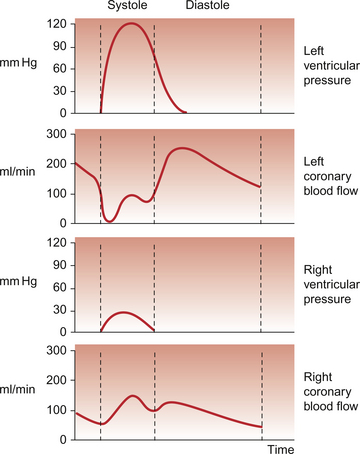
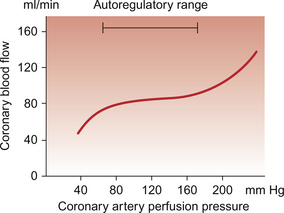
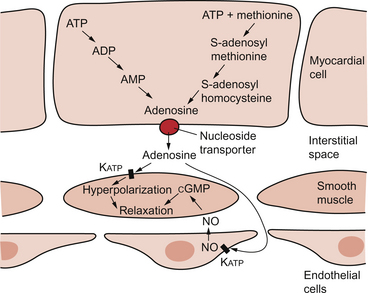
5 The term ‘coronary’ was first conceived to describe a crown-like arrangement of the arterial blood vessels supplying the heart muscle. If the heart is considered as an upside-down cone with the flat base placed at the level of the atrioventricular groove, then the coronary arteries may be visualized as a ring at the base of the cone with branches which pass towards the tip (Fig. 5.1). This network of coronary arteries arises from two main origins in adjacent aortic sinuses. The anterosuperior sinus gives rise to the right coronary artery (RCA). This passes down the anterior atrioventricular (AV) groove. It gives rise to marginal branches which supply the anterior free wall of the right ventricle (RV). At the junction between the anterior and inferior aspects of the RV it gives off a significant branch—the acute marginal artery. It then passes inferiorly and in 70% of subjects terminates in a right posterior descending branch which supplies the inferior aspects of the RV, left ventricle (LV) and interventricular septum. The sinoatrial node is supplied by a branch of the RCA in 60% of cases and from the circumflex artery in 40%. The AV node is supplied by the RCA in 90% of cases. Damage to vessels supplying portions of the conducting system may lead to specific defects. In the case of damage to the sinus nodal artery it may lead to ‘sick sinus syndrome’ in which the frequency of generation of cardiac action potentials becomes randomly variable and inappropriate (tachy-brady syndrome). Damage to the supply to the AV node or bundle of His may lead to complete heart block (see Chapter 3) and is particularly associated with inferior myocardial infarction. An imbalance between the oxygen demands of the heart and amount that can be supplied by the coronary blood supply leads to the development of an hypoxic pain originating in the heart which is called angina (see p. 55). An outline of a clinical case history is shown in Case 5.1:1. At rest the myocardium receives about 5% of cardiac output. In the normal ‘textbook’ person there is a potential for cardiac output to increase about fivefold during exercise (see Chapter 13). This is roughly paralleled by changes in coronary blood flow and the necessity for this is largely dictated by the high oxygen extraction rate of cardiac muscle. In skeletal muscle, under resting conditions, only of the order of 25–30% of the oxygen carried in arterial blood is extracted for use in the muscle (Fig. 5.2). The saturation of haemoglobin with oxygen in skeletal muscle therefore decreases from about 97–98% (arterial blood) to about 70% (venous blood). Even under resting conditions the venous drainage from cardiac muscle is only 25% saturated, meaning that of the order of 75% of the oxygen in arterial blood has been extracted and used metabolically. During exercise, increased oxygen delivery to contracting skeletal muscle can be provided by a combination of increased blood flow (see Chapter 13) but also by increased (up to 80–90%) extraction of oxygen from haemoglobin. In the heart as oxygen extraction at rest is already about 75% there is limited scope for increasing oxygen delivery by this route. Studies with 11C-acetate positron emission tomography (PET) scanning suggest that, in the heart, oxygen extraction from arterial blood can rise to 90% during exercise but even this is a limited way of increasing oxygen delivery. The bottom line is that if the heart needs increased oxygen supply it must be mainly provided by increased coronary blood flow. The corollary of this is that pathological mechanisms which impair coronary blood flow must limit cardiac performance. Coronary blood flow, particularly to the left ventricle, is particularly affected by the contraction of the myocardium which crushes coronary vessels (Fig. 5.3). This mainly affects blood vessels in the subendocardial layers of the heart muscle and the blood vessels on or close to the surface of the heart are relatively unaffected. The subendocardial layers are therefore more prone to ischaemic damage. In the left ventricle, because of the high pressures developed in the contracting ventricle, coronary blood flow is much higher during diastole than during systole. In the right side of the heart intraventricular pressures are lower and so the effect of ventricular systole on coronary blood flow is less marked. When heart rate increases during exercise the duration of diastole is shortened more markedly than the duration of systole. This imposes a limitation on increases in coronary blood flow and is probably the limiting factor on maximum exercise ability in normal individuals. Coronary blood flow is autoregulated (Fig. 5.4). This means that over a range of mean arterial pressures, probably in humans from about 50 to 120 mm Hg, coronary blood flow is relatively independent of arterial pressure. This is thought to result especially from responses of arterioles which are less than 150 μm diameter. Thus, as the arterial pressure increases through the autoregulatory range the smooth muscle in the wall of these arterioles contracts to maintain flow constant. The major regulatory factor determining coronary blood flow is myocardial oxygen demand coupled to the production of vasodilator metabolites. These metabolites particularly affect blood vessels in the 150–170 μm size range. The vascular smooth muscle is thought to be particularly sensitive to changes in [adenosine], [K+], [H+] and to local changes leading to an increase in interstitial osmolarity (see Chapter 9). A major part of this vasodilator action is mediated by the opening of ATP-sensitive K+ channels. This leads to hyperpolarization and consequently to relaxation of the smooth muscle. The source of the vasodilator adenosine has been a subject of conjecture. Berne (1980) proposed that it was produced under hypoxic conditions by the complete dephosphorylation of ATP. An alternative pathway was put forward by Deussen (1989), in which adenosine was formed from ATP via the intermediate formation of S-adenosyl methionine and S-adenosyl homocysteine. Both pathways are thought to contribute to interstitial [adenosine]. The following points seem to be relevant to understanding these events. ATP, ADP and AMP are all polar molecules as a result of ionization of their phosphate groups and will not easily cross cell membranes. Adenosine is non-polar and can leave the myocardial cell once it has been formed. Adenosine in the interstitium has a very short half-life (of the order of 10 s) and so must be continuously generated. [ATP] inside cells is about 5 mmol/L but interstitial [adenosine] is about 10 nmol/L, a 500 000-fold difference in concentration. Therefore, only a very small proportion of the intracellular ATP would need to be metabolized to provide relatively big changes in interstitial [adenosine] (Fig. 5.5). Endothelial influences on blood vessel diameter are described elsewhere (see Chapter 9). Increased shear stress on the endothelium leads to production of nitric oxide and thence to vasodilatation. This occurs particularly in large coronary arteries but it does not appear to contribute to the mechanism of autoregulation. Endothelial dysfunction leading to impaired nitric oxide release is a characteristic of a number of pathological conditions which will affect the coronary blood vessels including hypercholesterolaemia, atherosclerosis and hypertension. Assessment, prevention and treatment of endothelial dysfunction is emerging as an important area of clinical medicine, especially in relation to the coronary circulation. Modulation of coronary blood flow via the sympathetic nervous system primarily acts through α1-adrenoceptors on relatively large vessels. Vessels less than 100 μm diameter predominantly have α2-adrenoceptors but α1-receptors are also present. Activation of either of these populations of α-receptors leads to vasoconstriction and this is the dominant sympathetically mediated response. In the past there has sometimes been confusion about the role of β-receptor-mediated vasodilatation. Although such receptors do exist in limited numbers on coronary vessels, the vasodilator response which follows β-agonist infusion is mainly a result of increased metabolite (e.g. adenosine) generation following an increased force of ventricular muscle contraction (i.e. an inotropic response). Coronary vasodilatation directly as a result of β-adrenoceptor activation is a very minor component of coronary vascular control. The most frequent cause of obstruction in a main coronary artery is atherosclerosis. As this is not confined to the coronary circuit but may develop in any major vessels in the high-pressure arterial side of the circulation the details of the pathogenesis of atherosclerotic lesions are described in Chapter 8. Occlusion of the coronary arteries may become critical in various ways:
BLOOD SUPPLY TO THE HEART
Anatomy of the arterial supply and venous drainage of the Heart
Regulation of coronary blood flow
Ischaemic heart disease
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine