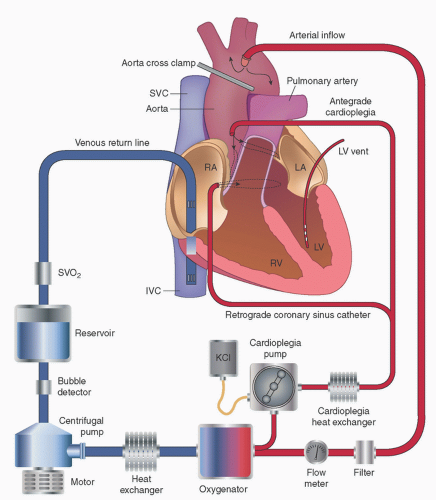
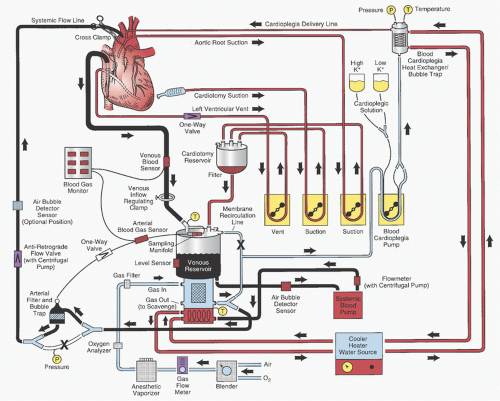
Ascending Aorta
In the early days of CPB, arterial inflow was through the subclavian or femoral artery (
79), but currently, as first proposed by Nunez and Bailey in 1959 (
80), it is usually through a cannula inserted into the ascending aorta The advantages of this approach over the femoral (or iliac arteries) (
Table 2.4) include ease, safety, and the fact that it does not require an additional incision. The surgical technique for aortic cannulation has been reviewed in detail by others (
9,
17,
81,
82). The site for cannulation is selected on the basis of the type of cannula to be used, the operation planned (i.e., how much of the
ascending aorta is available), the quality of the aorta, and the surgeon’s preference.
Atherosclerosis with or without calcification frequently involves the ascending aorta and poses problems with arterial cannulation and application of clamps and vascular grafts. Dislodgment of atheromatous debris either by direct mechanical disruption or from the “sand-blasting” effect of the jet coming out of the arterial cannula is thought to be a major cause of perioperative stroke (
83,
84,
85). Atherosclerosis is also considered a risk factor for perioperative aortic dissection (
86) and postoperative renal dysfunction (
87).
Traditionally, surgeons have relied on palpation to detect these changes and select sites for cannulation, cross-clamping, and so on, and this should continue to be one component of the evaluation of the aorta. Mills and Everson (
88) recommend using a 10- to 20-second period of venous inflow occlusion to reduce systemic arterial pressure to 40 to 50 mmHg to improve the reliability of palpation of the ascending aorta. However, palpation is much less sensitive and accurate than epivascular ultrasonic scanning (
89,
90,
91,
92). Details on how to perform an intraoperative epiaortic ultrasonographic examination are provided in the 2007 ASE/SCA guideline (
93). Unfortunately, TEE, which is more convenient, is not as sensitive because of limited views it can provide of the more distal ascending aorta where cross-clamping and cannulation are performed (
91,
92,
94). However, some believe it can be used as a screening method to determine which patients need epiaortic scanning. If no significant atherosclerosis is detected in the ascending, transverse, or proximal descending aorta, it has been suggested that epiaortic imaging is not necessary (
95), but others disagree (
95b).
Epiaortic and transesophageal scanning should be considered complementary (
92). Beique et al. (
85) suggested using epiaortic scanning in all patients who have a history of transient ischemic attacks, strokes, severe peripheral vascular disease, and palpable calcification in the ascending aorta, calcified aortic knob on chest X-ray, those older than 60 years, and those with TEE findings of moderate aortic atherosclerosis. Others advocate epiaortic scanning of the ascending aorta in all patients older than 50 years (
96). Three evidence-based guidelines recommend that “intraoperative TEE or epiaortic ultrasound scanning of the aorta should be considered (Class IIa, level of evidence B)” (
97), that “epiaortic ultrasound-guided changes in surgical approach… (provide) neuroprotection during CPB (IIb)” (
78), and that “routine epiaortic ultrasound scanning is reasonable to reduce the incidence of atheroembolic complications (Class IIa, level of evidence B)” (
98). If atherosclerosis is detected, then the sites for insertion of cannulas, grafts, and application of vascular clamps are modified. If extensive atherosclerosis precludes arterial cannulation in the ascending aorta, then the femoral route should be considered (see subsequent text). However, in this case, the transverse and descending aorta should be evaluated by TEE to rule out extensive atheroma that might be embolized into the brain or elsewhere with retrograde flow from a femoral cannula. If such is the case, then axillary-subclavian or innominate artery cannulation should be considered. Studies using historical control subjects suggest improved neurologic outcome with echocardiographic-based modification of surgical techniques in handling the ascending aorta (
85,
90,
99).
If atheroma is extensive in the ascending or transverse aorta, some clinicians have suggested using a long arterial cannula that is inserted in the ascending aorta and threaded around into the proximal descending aorta to reduce the “sand-blasting” effect (
100). Others have advocated doing an endarterectomy under deep hypothermic circulatory arrest (DHCA) if severely protruding or mobile atheromas are detected (
101), but in one study of 268 patients with severe protruding atheromas, aortic arch endarterectomy (in 43 patients) was associated with a higher stroke rate (35% vs. 12%) and mortality (19% vs. 12%) than when endarterectomy was not performed (
102). In addition, endarterectomy was found, on multivariate analysis, to be an independent predictor of stroke (Odds Ratio [OR] 3.6) (
103).
If the ascending aorta is totally calcified and rigid (so-called “porcelain” aorta), then entirely different strategies for cannulation and surgery must be used. These include no clamping of the ascending aorta, use of an alternate site for arterial cannulation, performing the operation “off-pump” if feasible, or, in selected cases, graft replacement or endarterectomy of the ascending aorta during DHCA (
96,
104). Unfortunately, graft replacement of the atherosclerotic ascending aorta is intrinsically a high-risk procedure (
105). If there is no intraluminal debris, Liddicoat et al. (
106) used an intraluminal balloon designed for port-access surgery, which is inserted through a purse-string suture in an atherosclerosis-free portion of the aortic arch to occlude the aorta. Others used a urinary (Foley) catheter in a similar manner (
107).
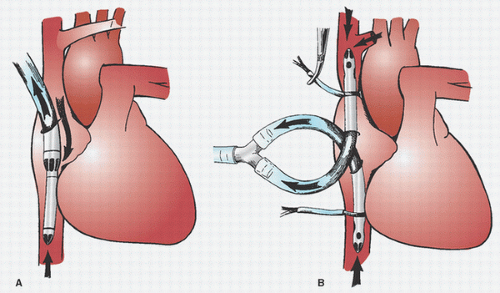
Cannulation of the Ascending Aorta
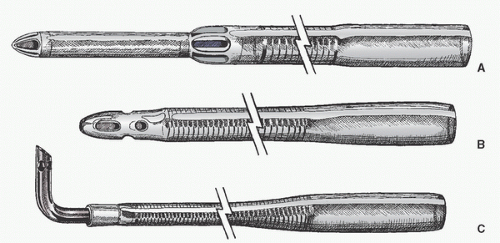
Many surgeons insert two concentric purse-string sutures into the aortic wall. Surgeons differ as to whether these should be shallow, deep, or full-thickness bites. Unal et al. (
108) discuss in detail the placement of the aortic purse-string suture and extol the virtues of a tangential suture technique (TST). Most surgeons then incise and dissect away the adventitia within the purse-string suture. Most avoid using a partial occluding clamp, except in pediatric patients, to minimize clamp trauma to the aorta. Optimal arterial blood pressure during cannulation (mean arterial pressure of approximately 70 to 80 mmHg, systolic pressure of approximately 100 to 120 mmHg) is probably important: if too high, there may be a greater chance of tears and dissection and blood loss and spray; if too low, the aorta tends to collapse, it is harder to make an incision and insert the cannula, and there is a greater risk of damaging the back wall of the aorta. An appropriately long full-thickness incision is then made, and the leak is controlled with a finger or by approximating the adventitia or by simultaneously inserting the cannula.
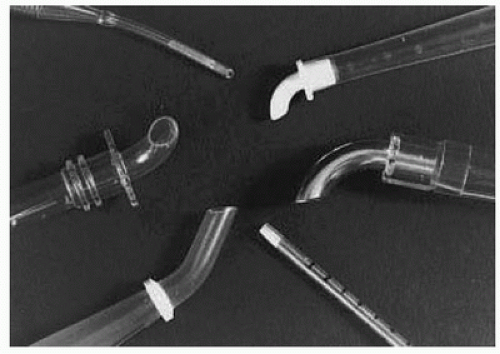
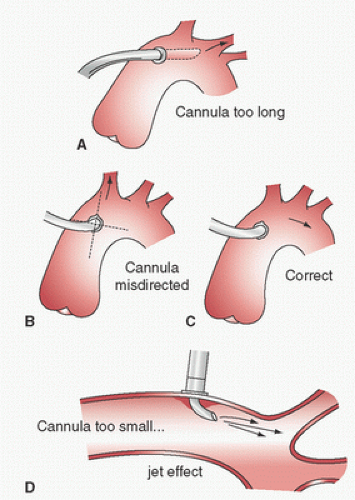
Dilators are sometimes used. If a right-angled cannula tip is used, it is often initially directed toward the heart and then rotated 180° to confirm intraluminal placement. Brief vigorous back bleeding out of the open cannula is then allowed to eliminate air or atheromatous debris and to further confirm intraluminal placement. This can be additionally confirmed by noting a pulsatile pressure approximating radial artery pressure in the CPB circuit arterial-line pressure monitor. Proper position of the cannula tip is critical. Most surgeons insert only 1 to 2 cm of the tip into the aorta and direct it toward the middle of the transverse arch to avoid entering the arch vessels (
Fig. 2.9). Grooters et al. (
61) point out that atherosclerosis is often more severe in the aortic arch, against which the jets from these short cannulas are directed, which may become the source of cerebral emboli, especially when the ascending aorta is relatively free of atherosclerosis. Barbut et al. (
109) noted high-grade plaque with greater frequency in the arch (18%) than in the ascending aorta (5.3%), and Weinstein (
63) has attributed the fact that more strokes occur in the left than the right cerebral hemisphere to jets striking atheroma in the arch. To minimize this risk, Grooters et al. (
61) have advocated directing the jet toward the ascending aorta (when it is free of atherosclerosis) and/or the use of dispersion type arterial cannulas. As mentioned earlier, others have advocated threading a long cannula into the proximal descending aorta to reduce the velocity and turbulence in the aortic arch to reduce the “sand-blast” effect and emboli (
100), although atheromata may be dislodged by the act of inserting this cannula through the intervening thoracic aorta. Mullges et al. (
110), in
a small RCT of 60 patients undergoing CABG, found that using an elongated cannula with the tip in the descending aorta, as compared with a short cannula in the ascending aorta, was associated with fewer microembolic signals (TCD), but there was no difference in cognitive performance (seven neuropsychological tests) 9 days postoperatively.
After the arterial cannula is inserted, a test infusion with the systemic pump through the arterial line before initiating CPB is recommended (regardless of location of the arterial cannula, i.e., ascending aorta/arch, femoral artery, axillary/subclavian, etc.). A higher-than-expected pressure in the circuit arterial line warns of possible dissection and may help avoid a more extensive dissection. Another method to assess this was described by DeBois and colleagues (
111). The lack of negative flow or a flow of <500 mL/min during retrograde arterial priming suggests cannula misplacement or occlusion.
Complications of aortic root cannulation include inability to introduce the cannula (interference by adventitia or plaques, too small an incision, fibrosis of the wall, low arterial pressure); intramural placement; dislodgment of atheroemboli, air embolism from the cannula, injury to the back wall of the aorta; persistent bleeding around the cannula or at the site after its removal; malposition of the tip (
Fig. 2.9), or also to a retrograde position possibly even across the aortic valve, against the vessel wall, or into the arch vessels; abnormal cerebral perfusion; obstruction of the aorta in infants; aortic dissection; and high CPB arterial-side line pressure. High CPB circuit arterial-line pressure may be a clue to malposition of the tip against the vessel wall or into an arch vessel, cannula occlusion by the aortic cross-clamp, aortic dissection, a kink in the inflow system, an arterial-line clamp that is still on, or the use of too small a cannula for the intended CPB flow.
Inadvertent cannulation of the arch vessels or directing the jet into an arch vessel may cause irreversible cerebral injury and reduced systemic perfusion. Suggestive evidence includes high systemic line pressure in the CPB circuit; high pressure in the radial artery if supplied by the inadvertently cannulated vessel (or low pressure if not supplied by the cannulated vessel); unilateral facial blanching when initiating bypass with a clear priming solution; asymmetric cooling of the neck during perfusion cooling; and unilateral hyperemia, edema, petechiae, conjunctival tearing, or dilated pupils. Before CPB, palpation of the carotid arteries may reveal asymmetric pulsation (reduced on the cannulated side) and the opposite may be observed during pulsatile bypass (increased pulsation on the cannulated side). Before CPB, the radial artery catheter may reveal sudden damping if the cannula is inserted in the arch vessel supplying the monitored radial artery.
It has been suggested that the Coanda effect (in which a jet stream adheres to the boundary wall and hence produces a lower pressure along the opposite wall) may be associated with carotid hypoperfusion (
112). This has been shown experimentally and may account for some cerebral dysfunction after CPB using aortic cannulation. Salerno et al. (
113) detected major electroencephalographic abnormalities due to malposition of a cannula in 3 of 84 patients undergoing arch perfusion, possibly on the basis of the Coanda effect. Recently, a number of groups have studied this in mock circulations. Kaufmann and colleagues (
114) studied the impact of cannula position on flow distribution utilizing CFD validated by particle imaging velocimetry. They found that direction of the cannula jet and its distance from a branch vessel could result in localized retrograde flow from a Venturi effect. Tokuda and colleagues (
115) have also used CFD to analyze blood flow in the aortic arch during CPB, and Menon et al. (
116) showed that neonatal cannula orientation could induce backward flow due to Venturi effect.
Antegrade aortic dissection (
Table 2.5) associated with ascending aortic cannulation has been reported in 0.01% to 0.09% of cases (
82,
86,
117,
118,
119,
120). Aortic dissection should be suspected when any of the following are observed: a sudden decrease in both venous return and arterial pressure, excessive loss of perfusate, increased circuit arterial-line pressure, evidence of decreased organ perfusion (oliguria, dilated pupil, electroencephalographic changes, electrocardiographic evidence of myocardial ischemia), blue discoloration of the aortic
root (because of intramural hematoma), and bleeding from needle or cannulation sites in the aortic root. Subadventitial hematomas tend to be less extensive and softer, and usually resolve when incised. TEE and/or epiaortic ultrasound scanning are useful in diagnosing aortic dissection (
93,
119,
120,
121,
122).
Gott et al. (
118) and Still et al. (
117) have discussed iatrogenic aortic dissection in detail. Management usually involves prompt cessation of CPB, recannulation distal to the dissection (usually femoral but occasionally into the distal aortic arch), induction of deep hypothermia, and a period of circulatory arrest while the aorta is opened and the extent of the injury analyzed and repaired by direct closure, use of a patch, or replacement of the ascending aorta with a tubular graft. Occasionally, small injuries can be repaired off CPB by closed
plication (
118), but such repairs may fail early or later and therefore graft replacement is generally favored (
124,
120). Survival of those cases recognized and treated in the operating room has ranged from 66% to 85%. When not recognized until postoperatively, survival has been 50% or less (
Table 2.5).
False aneurysms, which may rupture or become infected, are late complications of aortic cannulation (
123,
124). In a review of the literature (
123) and the experience of a single institution (
124), the arterial cannulation site was found to be the source of approximately one third of the ascending aortic aneurysms that follow cardiac surgery, of which approximately 40% were infected. The mortality of such complications was approximately 50%.
Cannulation of the femoral or iliac arteries (exposed through a retroperitoneal suprainguinal approach) is indicated when there is an aneurysm of the ascending aorta or when it is otherwise unsatisfactory for cannulation. This may also be indicated when there is inadequate space available due to multiple procedures involving the ascending aorta, for peripheral cannulation under local anesthesia in unstable patients, during reoperations prophylactically, when bleeding complications occur during reentry, or when an antegrade dissection complicates aortic cannulation. Femoral cannulation requires a second incision and limits the size of the cannula that can be used. Hence, the adverse consequences of fluid jetting effects and high pressure gradients are more likely. Lees et al. (
125) found no difference in the distribution of blood flow and vascular resistance between retrograde (femoral artery infusion) and antegrade (aortic root infusion) flow in monkeys. Shann and Melnitchouk (
10) made recommendations for size and model and maximal flow rates of various femoral artery cannulas based on patient’s size and these are summarized in
Table 2.6.
Femoral cannulation is associated with many complications (
23,
113,
126) including trauma to the cannulated vessel, such as tears, dissection, late stenosis or thrombosis, and bleeding; lymph fistula; infection; embolization; and limb ischemia. Muhs et al. (
127) reported their experience with five (0.7% incidence) arterial injuries early (<30 days) following femoral perfusion utilizing the port-access system in 739 patients. Because the retrograde perfusion cannula usually totally occludes the direct blood supply to the cannulated limb, ischemic complications (acidosis, compartment syndrome, muscle necrosis, and neuropathy) may develop if cannulation exceeds 3 to 6 hours (
128,
129,
130). The risk of distal ischemia can be minimized by placing a Y-connector or Luer-lock port in the arterial line and attaching a smaller cannula (e.g., 8-14F pediatric arterial cannula) which is then inserted distally through the same arteriotomy (
130) or an 8.5F introducer catheter inserted into the distal superficial femoral artery using the Seldinger technique (
131) to maintain perfusion of the leg. Alternately, VanderSalm (
132) advocated suturing a 10-mm polytetrafluoroethylene graft end-to-side on the common femoral artery into which the 24F femoral cannula is inserted. This latter technique not only prevents lower extremity ischemia but also may reduce risk of arterial injury and retrograde dissection. Use of a coated Dacron graft may be associated with less bleeding. If distal limb perfusion is used and the ipsilateral femoral vein has also been cannulated, then a method to provide better venous drainage of that limb is suggested to reduce edema. Edema can be minimized either by not taping the vein around the cannula (
130) or by placing a second (12F) venous cannula through the saphenous vein into the distal femoral vein (
133). If limb ischemia does occur, Beyersdorf et al. (
134) described a method of controlled limb reperfusion to improve outcome.
Femoral perfusion may lead to cerebral and coronary atheroembolism if there are extensive atheromas in the aortic arch or descending aorta; ideally, this should be assessed by TEE before selecting the femoral route. If severe atherosclerosis is present, an alternate route should be used if possible. Femoral perfusion may also aggravate preexisting aortic dissections, and an alternate site for cannulation (see subsequent text) is recommended by some authors (
135).
The most serious complication of femoral cannulation is retrograde arterial dissection, which may lead to retroperitoneal hemorrhage or retrograde dissection extension all the way to the aortic root. The incidence of this complication has been reported at between 0.2% and 1.3% (
136,
137,
138,
139,
140,
141), although rates as high as 1 in 30 (3%) and 2 in 51 (4%) (
142,
143) and as low as 0 in 702 (
113) have been reported. Kay et al. (
136) noted a rate of 3% in 378 patients older than 40 years. Femoral cannulation is being more frequently used during
limited-access surgery and has been complicated by fatal dissection (
143,
144,
145). Galloway et al. (
141) reported an arterial dissection rate of approximately 0.8% in a registry of 1,063 patients undergoing retrograde femoral cannulation and CPB with a port-access system (HeartPort, Inc., Redwood City, CA), whereas Grossi et al. (
145b) reported a rate of 0.3% in a single-center experience with 714 patients undergoing minimally invasive mitral valve surgery. (In 564 of these patients, arterial cannulation was into the femoral artery.)
Retrograde arterial dissection is thought to be caused by either direct (cannula) or indirect (jet) trauma and to be more likely in the presence of atherosclerosis or cystic medial necrosis and in patients older than 40 years (
126,
136). Retrograde aortic dissection may present like antegrade aortic dissection already described, but may be more difficult to recognize if it does not extend into the ascending aorta. In these cases, it may present only as a sudden decrease in venous return and arterial pressure, excessive loss of perfusate, increased circuit arterial-line pressure, and oliguria. In this situation, TEE scanning of the proximal descending aorta is extremely helpful in making the correct diagnosis.
Because of the nature of the dissection and the flap, discontinuation of retrograde perfusion and resumption of antegrade flow (through a cannula in the ascending aorta or by normal cardiac function) may resolve the problem. This may permit different management from antegrade dissections. If the dissection occurs early in the procedure, simply discontinuing CPB immediately (and hence retrograde femoral perfusion) and restoring intravascular volume (which can be facilitated by attaching the arterial line to the venous cannula and infusing residual blood from the extracorporeal circuit) and then aborting the planned operation and doing nothing further to the ascending aorta, even if it is affected by the dissection, can be successful (
138). If the dissection occurs later, when it is not possible or desirable to come off CPB, retrograde perfusion is immediately discontinued and the arterial cannula is introduced into the true lumen in the ascending aorta (often through the false lumen). Bypass is then resumed with antegrade perfusion and the planned operation may sometimes be completed without repair of the dissection itself or the ascending aorta. Carey et al. (
140) reported long-term success in six of seven patients using this approach. Others recommend graft replacement of the ascending aorta (
139,
146).
There is particular concern about the use of the femoral artery for arterial infusion during the repair of spontaneous (type A or B) aortic dissection because of the risk of malperfusion (
135,
147,
148), and for this reason use of the axillary artery is often recommended. However, others have reported good results utilizing femoral cannulation in this circumstance. Fusco et al. (
149) and Shimokawa et al. (
150) reported malperfusion requiring change in cannulation after starting out with femoral cannulation in only 2/79 and 3/107 attempts, respectively. Dhareshwar et al. (
151) have also found femoral artery cannulation safe for surgery in cases of acute type A dissections. On the other hand, Voci et al. (
152) used sonicated albumen in 27 cases of type A dissections to determine which lumen was perfused with femoral artery infusion. In 13 cases (48%) only the true lumen was perfused, whereas in 11 (41%) both lumens were perfused, and in 3 (11%) only the false lumen was perfused. In the latter three cases, this was corrected by cannulating the other femoral artery. Interestingly, the false lumen was partially or completely perfused in 13 of 19 (68%) cases when the left femoral artery was cannulated, and in only 1/8 (13%) when the right femoral artery was cannulated. Unfortunately, the strength of the pulse has not been helpful in deciding which femoral artery to perfuse into. Orihashi et al. (
153) found evidence of a new dissection in the abdominal aorta by TEE (loss of flow in celiac or superior mesenteric arteries or appearance of a flap) in 3 of 11 patients with acute (
5) or chronic (
6) dissecting aortic aneurysms (DAA) which had not previously involved the abdominal aorta. All new dissections occurred at some delay after initiation of perfusion and were associated with metabolic acidosis that resolved with antegrade perfusion. These studies provide further evidence of the liability of femoral artery inflow in patients with aortic dissections.
Use of the axillary artery (either by direct cannulation or through an attached 8-mm graft) instead of the femoral artery when ascending aortic cannulation is infeasible or undesirable is increasingly advocated (
155,
156,
157,
158,
159) (
Fig. 2.10). During a left thoracotomy, the intrathoracic subclavian artery may be cannulated (
160). The putative advantages of the axillary artery over the femoral artery include lower likelihood of atherosclerosis, better collateral flow with lower risk of ischemic complications, and better healing with fewer wound complications. By avoiding retrograde descending thoracic and abdominal aortic flow, it is also less likely to cause cerebral athero-embolization. Hedayati et al. (
161) demonstrated in an animal model that axillary cannulation reduced cerebral microemboli compared with aortic cannulation. Kaufmann and colleagues (
114,
162), utilizing CFD, found that cannulation of the right subclavian artery provided better flow into the arch vessels than direct aortic cannulation as long as the cannula tip was sufficiently far away from the origin of the right vertebral artery (otherwise, it could cause retrograde flow in that vessel).
Some advocate axillary artery cannulation for type A aortic dissections, because it is thought to be less likely to result in malperfusion and further expansion of the dissection, as may occur with femoral arterial perfusion (
135,
147,
148). In this situation, some favor use of the right axillary artery (
135) while others favor the left side (
148). Adequate arch
vessel hydrodynamics have been demonstrated during perfusion through the right subclavian artery in a mock circulation (
163); however, the absence of subclavian artery stenosis should first be documented by comparing noninvasive or invasive arterial pressure in each arm (
164) before choosing this route.
The axillary artery is approached through a 4- to 10-cm incision below and parallel to the lateral two thirds of the clavicle, or in the deltopectoral groove (
157,
165). Care must be taken to avoid traction on the brachial plexus. The axillary vein is retracted away from the artery (but may be used for venous cannulation) (
156). A purse-string suture may then be placed in the axillary artery and a 20 to 22F right-angled or flexible arterial cannula is inserted in a retrograde direction 2 to 3 cm. In this circumstance the contralateral radial or brachial artery (usually the left) must be used for intra-arterial pressure monitoring. Alternately, an 8- to 10-mm nonporous graft may be sewn end-to-side to the axillary artery (
157) and the perfusion cannula inserted only
partially into this graft or the arterial line from the ECC is connected directly into this graft via a °inch (into an 8-mm graft) or 3/8 inch (into a 10-mm graft) connector. This maintains the functionality of ipsilateral radial artery pressure monitoring. An advantage to cannulating the right axillary artery is that subsequent to occlusion of the innominate artery this provides a route for administering antegrade arterial cerebral perfusion (at least partial, through the right carotid and vertebral arteries) during DHCA for surgery involving the aortic arch. In this circumstance, some practitioners also selectively perfuse (antegrade) the left common carotid artery as well (
166). If a sidearm graft is used for cannulation, then monitoring the pressure in the right radial artery provides a clue to cerebral perfusion pressure during antegrade cerebral perfusion. During lateral thoracotomies, either the axillary artery may be approached via the axilla through a vertical incision along the lateral border of the pectoralis major muscle (
156) or the subclavian artery may be cannulated intrathoracically.
Many reports of the use of the subclavian artery in small series have observed a low incidence of complications (see summaries by Fusco et al. (
149) and Schachner et al. (
167)), but four larger series of a total of 823 cases (two with 284 and 399 cases, respectively (
168,
169)) have painted a more realistic picture (
167,
168,
169,
170). Axillary artery injury, thrombosis, or dissection occurred in 12 patients, brachial plexus injury in 9, new aortic dissection in 5, malperfusion in 3/65 patients (but all among the 35 undergoing repair of acute type A DAA) in one series (
167), and ischemia or compression syndrome in the arm in 4. On 17 occasions in three series (approximately 4%), the authors reported that they were unable to perfuse through the axillary artery (nine due to poor back bleeding or high resistance, four due to the development of local dissection, three due to a small artery or unusual anatomy, and one because it was involved by a chronic dissection). On the other hand, no local wound problems were encountered. The right subclavian/axillary artery was used in the vast majority of cases (97%). Direct cannulation was employed in 77% and a side graft in only 23%, although perfusion through a side graft is favored by several authors (
168,
169,
170,
171). These authors believe that this approach minimizes the risks of arterial injury, inadequate flow, dissection, and compartment syndrome, and better enables cerebral perfusion pressure monitoring through the right radial artery during selective antegrade arterial cerebral perfusion. A propensity score analysis by the Cleveland Clinic Group found a lower rate of arterial injury and aortic dissection when a side-arm graft was employed (
169). However, all three cases of malperfusion from Schachner et al. occurred when a side graft was employed (
167). The latter group had to switch from the use of subclavian perfusion (to aorta in two and femoral artery in five) in 7/65 attempts due to malperfusion in three, inadequate flow in two, and local dissection and aortic dissection in one each (
167). The Mount Sinai Medical Center group in New York City favors direct cannulation because it is less time-consuming, lowers the risk of bleeding, is technically easier to perform, and is not associated with hyperperfusion of the cannulated arm. They have used direct cannulation with a 20 to 26F wire-reinforced right-angled cannula (Edwards Life Science, Irvine, CA) in all of their 284 patients (
168). Schachner et al. (
167) considers the choice of direct cannulation versus a side graft to still be a matter of debate.
At least three case reports of aortic or innominate dissections associated with use of the subclavian arteries for arterial inflow have appeared (
172,
173,
174) and were reported in 4/539 patients (0.7%) in three large series (
167,
169,
170). This complication is likely less common when a side graft is employed (
169) but indicates the need to carefully monitor for and be suspicious of this possibility (
165,
172,
174).
Despite the reputed advantage of a lower risk of malperfusion using the right axillary/subclavian artery (compared with the use of femoral artery) when operating on acute type A aortic dissections (
135,
147,
148,
165), as mentioned earlier others have reported favorable results using the femoral artery in this circumstance (
149,
150,
151). Furthermore, malperfusion was encountered in 3 of 37 cases in one series in which the subclavian artery was used for arterial inflow (
167), although this complication was not reported in four other reports of the use of right subclavian artery in a total of 132 acute type A dissecting aortic aneurysms (
165,
169,
170,
171).
Dhareshwar et al. (
151) assert that “the benefits of using the axillary artery as opposed to the femoral artery for cannulation have yet to be proven conclusively,” and believe that the use of cerebral monitoring to identify malperfusion is more important than the site of arterial cannulation. Fusco (
149) and Shimokawa (
150) also emphasize the need to monitor for malperfusion regardless of the vessel chosen for arterial cannulation and recommended monitoring bilateral radial artery pressures, use of TEE (size of the true lumen and flow into the arch vessels), and palpation of the aorta. Estrera and colleagues found monitoring with power M-mode multichannel TCD helpful in this regard (
175), and others have used two-channel TCD, near-infrared spectroscopy (NIRS) (i.e., bilateral cerebral oximetry) (
176,
177), electroencephalography (EEG), and jugular venous oxygen monitoring for this purpose.
In a systematic review of the literature, Gulbins and Colleagues (
178) concluded that there was a trend toward improved neurologic outcome when the axillary artery was used as compared to the femoral artery, but this conclusion was weakened by the lack of any RCT and by the low number of patients compared. In a recent invited commentary, Geirsson concluded that this debate is far from resolved and opined that an RCT will never be possible (
179). Di Eusanio and colleagues (
180) compared their aortic arch surgery results in 200 patients utilizing central cannulation (right axillary in 128, innominate artery in 26, and ascending aorta in 46) to 273 patients utilizing femoral cannulation with propensity score analysis. They found a similar risk of postoperative death and permanent neurologic dysfunction in the two groups. Etz et al. (
181) compared their experience with antegrade perfusion (via the right axillary artery in 297 and direct aortic cannulation in 15) with retrograde perfusion (via the femoral artery) in 90 patients undergoing repair of acute Type A dissection. The incidence of early complications (mortality and postoperative stroke) was no different in the two groups but survival at 10 years was greater (71% compared with 51%) when antegrade perfusion was used.