(1)
Project-team INRIA-UPMC-CNRS REO Laboratoire Jacques-Louis Lions, CNRS UMR 7598, Université Pierre et Marie Curie, Place Jussieu 4, 75252 Paris Cedex 05, France
Abstract
“…there is a continuous and uninterrupted movement of blood from the heart through the arteries to the body as a whole, and likewise back from that body as a whole through the veins to the heart, with such flow and ebb that in such quantity and amount that it must somehow move in circle.” (Aletter from W.Harvey to C.Hofmann[1])
“… there is a continuous and uninterrupted movement of blood from the heart through the arteries to the body as a whole, and likewise back from that body as a whole through the veins to the heart, with such flow and ebb that in such quantity and amount that it must somehow move in circle.” (A letter from W. Harvey to C. Hofmann [1])
Blood is a major tissular component of the closed circulatory system, which supplies nutrients and oxygen using hemoglobin-containing red blood capsules to the body’s cells and removes metabolic wastes. This specialized liquid is composed of cells suspended in a liquid, the plasma (mostly water). It flows throughout the body in blood vessels due to a pressure difference set by the heart pump.
Blood cells are involved in tissue adaptation to hypoxia via angiogenesis as well as treatment of injury via blood coagulation and healing and other types of tissue damage, such as that caused by infection, by triggering inflammation and immunity. Carried cells, like vessel wall cells, sense and respond to mechanical stresses exerted by the flowing blood.
Blood is propelled throughout the circulatory system by the heart. Blood is ejected from the left and right cardiac ventricles into the systemic and pulmonary circulation, respectively. In the heart, nodal myocytes of the natural pacemaker — the sinoatrial node — spontaneously and rhythmically generate an electrochemical wave, or the so-called action potential, which characterizes heart automatism. This action potential then propagates down to other atrial and ventricular nodal cells and cardiomyocytes. The latter also propagate the action potential and contract to propel the blood. Coupling of (1) generation and propagation of the action potential (heart electrical activity); (2) cardiac wall contraction and deformation (solid mechanics aspects of heart activity); and (3) blood filling in and ejection out of both coupled ventricular pumps associated with closing and opening of auriculoventricular and ventriculo-arterial valves and myocardium perfusion is a non-trivial task. This problem has thus been tackled by splitting and separately processing the major processes. Today, accumulated knowledge, appropriate modelings, and updated technologies allow their coupling.
The cardiac pump sets up a pressure wave that impinges strongly upon walls of adjacent elastic arteries, which are then capable of ensuring a blood flow during the ventricular diastole. The blood vessel wall is a living tissue that quickly reacts to loads applied on it by the flowing blood. In any segment of the vasculature, endothelial and smooth muscle cells sense space and time variations in small-magnitude wall shear stress and large-magnitude wall stretch generated by the flowing blood. These cells respond with a short time scale (from seconds to hours) to adapt the vessel caliber according to the loading, especially when changes exceed the limits of the usual stress range. The mechanotransduction pathways determine the local vasomotor tone and subsequently the lumen bore of the reacting blood vessel. This regulatory mechanism is much faster than the nervous and hormonal control. It sets up the level of local resistance to blood flow: (1) in large arteries, which ensure the blood distribution in the body, to limit the cardiac postload; (2) in small resistive arteries, which irrigate the body’s tissues, to maintain the flow rate; and (3) in arterioles, which perfuse a cell population, to adapt the blood supply to metabolic needs of active cells.
1.1 Blood Functions
The blood performs 3 major functions: (1) transport through the body; (2) regulation of bulk equilibria; and (3) body immune defense against foreign bodies.
Blood supplies oxygen, hence energy, and conveys nutrients (vitamins, mineral ions, glucose, amino acids, fatty acids, among other glucids, protids, and lipids) to the tissues and removes carbon dioxide and waste products of cell metabolism toward lungs and purification organs. Kidneys filter blood. Toxins are not only removed in urine, but also by sweating. Blood transmits metabolic factors and messengers such as hormones to target organs.
Blood volume and electrolyte concentration are regulated. Blood maintains the body temperature (36.4–37.1 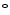 C) and acid–base equilibrium, controlling blood pH that remains in the range 7.35 to 7.45.1 Blood circulation transports heat throughout the body, thereby contributing to the thermoregulation. Increased blood flow at the body’s surface during warm weather or exercise permits higher heat loss. Conversely, when the ambient temperature decays, blood flow near the skin lowers to prevent heat loss.
C) and acid–base equilibrium, controlling blood pH that remains in the range 7.35 to 7.45.1 Blood circulation transports heat throughout the body, thereby contributing to the thermoregulation. Increased blood flow at the body’s surface during warm weather or exercise permits higher heat loss. Conversely, when the ambient temperature decays, blood flow near the skin lowers to prevent heat loss.
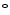 C) and acid–base equilibrium, controlling blood pH that remains in the range 7.35 to 7.45.1 Blood circulation transports heat throughout the body, thereby contributing to the thermoregulation. Increased blood flow at the body’s surface during warm weather or exercise permits higher heat loss. Conversely, when the ambient temperature decays, blood flow near the skin lowers to prevent heat loss.
C) and acid–base equilibrium, controlling blood pH that remains in the range 7.35 to 7.45.1 Blood circulation transports heat throughout the body, thereby contributing to the thermoregulation. Increased blood flow at the body’s surface during warm weather or exercise permits higher heat loss. Conversely, when the ambient temperature decays, blood flow near the skin lowers to prevent heat loss.Blood participates in the body’s defense against infection, as it transports immunocytes and antibodies, as well as in repair after injury. Innate immunity yields the first stage of defense against invading pathogens and signals for the development of adaptive immunity.2
Blood limits its own loss through damaged vessel walls by blood coagulation, or clotting (Chap. 9). Blood coagulation is a component of hemostasis, i.e., cessation of blood flow through a damaged vessel wall. Clot formation covers the injury site by a platelet and fibrin-containing material to stop hemorrhage and begin repair of the damaged vessel. Platelets immediately form a plug (primary hemostasis) that is simultaneously reinforced by fibrin strands, which result from the coagulation cascade of activation of coagulation factors (or clotting factors; secondary hemostasis).
Blood contains living cells and plasma (Table 1.1). Eight to twelve hours after a meal, 100 ml of blood contains 19 to 23 g of solids and 77 to 81 g of water. Blood cells include red blood cells (RBC), or erythrocytes, white blood cells (WBC), or leukocytes, and platelets, or thrombocytes.
Table 1.1
Blood composition and main characteristics in healthy adult male. The blood cells include erythrocytes (red blood cells), leukocytes (white blood cells), and platelets. Leukocytes are divided into 5 classes based on morphological and tinctorial characteristics. Neutrophils, eosinophils, and basophils are known as granulocytes due to granules in the cytoplasm. Monocytes and lymphocytes are involved in the body scavenging and defense. The blood plasma consists of water (90%), the remainder beingelectrolytes (sodium [Na + ], 142 mmol/l; chloride [Cl − ], 102 mmol/l; and potassium [K + ], 5 mmol/l), carbohydrates, lipids, and amino acids, etc.
Erythrocytes | 4.5– 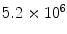 /mm3 /mm3 |
|---|---|
Hematocrit | 41–47% |
Leukocytes | 4–10 ×103/mm3 |
Neutrophils | 40–70% |
Eosinophils | 1–2% |
Basophils | 0.5–1% |
Lymphocytes | 20–40% |
Monocytes | 2–10% |
Platelets | 2– 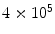 /mm3 /mm3 |
Ions | 295–310 mEq/l |
Protids | 70–80 g/l |
Lipids | 5–7 g/l |
Glucids | 0.8–1.1 g/l |
pH | 7.39–7.41 |
Osmotic pressure | 280–300 mosm |
Leukocytes operate in immunity. Five main classes of leukocytes exist: 3 types of granulocytes that have about the same size — neutrophils, eosinophils, and basophils — and 2 types of agranular leukocytes — lymphocytes and monocytes. Leukocyte lifespan in blood is several days.
1.2 Plasma
Plasma constitutes a body fluid subcompartment. The body fluids indeed form several compartments. The 2 major compartments include intra- and extracellular fluids. The extracellular fluids comprise: (1) interstitial fluids;3 (2) plasma; and (3) minor components, such as lymph,4 cerebrospinal fluid, digestive secretions, aqueous humor, and pleural, pericardial, and synovial fluids for pleura, pericardium, and joint lubrication. About 64% of body water is found in the intracellular space, about 25% in the intertitium ( ∼ 75% of extracellular liquid, about 8% in the plasma, and about 3% in the minor compartments (Table 1.2).
Table 1.2
Approximative water content of body fluid compartments (l).
Intracellular space | 27–30 |
|---|---|
Extracellular space | 14–17 |
Interstitial fluid | 11–13 |
Plasma | 3–4 |
Total body water | 41–47 |
1.3 Plasma Constituents
Plasma represents approximately 55% of the blood volume. The remaining is hematocrit (Ht), i.e., percent of packed cells5 (Ht 38–46% in women, 42–53% in men). Plasma is mainly composed of water, a suspending fluid (or solvent) for various solutes (Table 1.3). Plasma contains 92% water, 8% proteins ( ∼ 7 g/dl), and other substances.
Table 1.3
Plasma approximate composition (%). Plasma, the suspending fluid for peripheral blood cells, is composed of water, electrolytes, proteins (albumins 60–80%, globulins 16–36%, and fibrinogen 4%), amino acids, lipoproteins, other lipids, and glucids. Hormones, vitamins, and enzymes are conveyed by blood. The normal plasma volume is 40 to 90 ml/kg of body weight.
Water | ∼ 92 |
Proteins | ∼ 7.0 |
Electrolytes | ∼ 0.9 |
Lipids | ∼ 0.6 |
Glucids | ∼ 0.1 |
1.3.1 Electrolytes
Electrolytes, or ions, contribute to theosmotic pressure (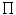 ), which is mainly regulated by the kidneys. Major electrolytes are Na + , K + , Ca2 + , Mg2 + cations, and HCO3 − , Cl − , HPO4 2 − , and SO4 2 − anions.
), which is mainly regulated by the kidneys. Major electrolytes are Na + , K + , Ca2 + , Mg2 + cations, and HCO3 − , Cl − , HPO4 2 − , and SO4 2 − anions.
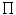 ), which is mainly regulated by the kidneys. Major electrolytes are Na + , K + , Ca2 + , Mg2 + cations, and HCO3 − , Cl − , HPO4 2 − , and SO4 2 − anions.
), which is mainly regulated by the kidneys. Major electrolytes are Na + , K + , Ca2 + , Mg2 + cations, and HCO3 − , Cl − , HPO4 2 − , and SO4 2 − anions.Cations and anions are unevenly distributed in body fluid compartments (Table 1.4). Sodium ion (Na + ) is the major cation and chloride (Cl − ) the major anion outside the cell. Inside the cell, potassium ion (K + ) is the major cation and phosphate (HPO4 2 − ) the major anion. At physiological pH, proteins are negatively charged.
Table 1.4
Distribution of ions and proteins (mEq/l) in intra-, and extracellular spaces, and plasma.
Cell | Intertitium | Plasma | |
|---|---|---|---|
Sodium (Na + ) | 10–15 | 130–150 | 135–145 |
Calcium (Ca2 + ) | 10 − 4 | 2–5 | 5–10 |
Potassium (K + ) | 135–150 | 4–5 | 3–5 |
Magnesium (Mg2 + ) | 30–35 | 1–3 | 1–3 |
Cations | 180 | 152 | 155 |
Chloride (Cl − ) | 3–9 | 108–125 | 100–108 |
Bicarbonate (HCO3 − ) | 10–12 | 27–30 | 23–28 |
Phosphate (HPO4 2 − ) | 40–80 | 2–3 | 1–3 |
Sulfate (SO4 2 − ) | 20 | 1 | 1 |
Proteins | 35–55 | ∼ 0 | 14–16 |
Organic acid | 20 | 5 | 5–6 |
Anions | 180 | 152 | 155 |
Ion transport through the cell membrane between the body fluid compartments requires specialized plasmalemmal proteins (Fig. 1.1 and Vol. 3 – Chaps. 2. Membrane Ion Carriers to 5. Receptors of Cellular Trafficking). Ion displacements in the different compartments affect the blood volume.
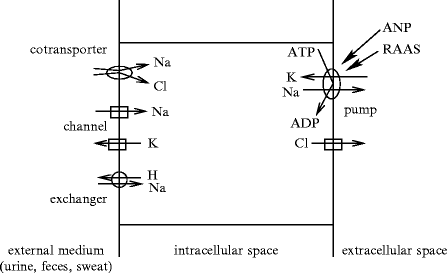

Fig. 1.1
Types of ion motions in the 3 main fluid compartments, extracellular (particularly the blood), intracellular, and external media
1.3.2 Glucids
Glucids are composed of: (1) oligosaccharides (glucose, fructose, and galactose); (2) disaccharides (saccharose, lactose, and maltose); and (3) polysaccharides (glycogen). Glucose and fructose can be used by cells. Other glucids require degradation into glucose. Glucids are important nutrients because they are energy sources.
Glycemia ( ∼ 1 g/l) is the blood glucose concentration, which depends on the exogenous supply and degradation of hepatic glycogen.6 Glycemia is stabilized by 2 pancreatic hormones,insulin andglucagon. Insulin decreases glucose level by cell use and storage, especially in the liver and the muscles. Glucagon increases glucose concentration. When glycemia falls below 70 mg/dl, parasympathetic nerves are stimulated and hormones are released (adrenaline, cortisol, glucagon, and growth hormone) to limit glucose uptake. On the other hand, hyperinsulinemia occurs to compensate for insulin resistance.
1.3.3 Plasma Proteins
Serum is plasma withoutfibrinogen (195–365 mg/dl) and other clotting factors. Fibrinogen acts on erythrocyte aggregation,7 hence influencing blood rheology (Vol. 7), and blood coagulation (Sect. 9.8). Main non-protein nitrogens (NPN) are urea, uric acid, creatine, creatinine, ammonium salts, and amino acids.
Serum proteins are composed of albumin and globulins (Table 1.5). Plasma proteins are responsible forosmotic pressure that maintains fluid balance across capillaries ( ∼ 3.3 kPa [ ∼ 25 mmHg]; Vol. 6 – Chap. 4. Cardiovascular Physiology).
Table 1.5
Plasma protein composition (%).
Albumin | ∼ 0.60 |
|---|---|
α1-Globulin | ∼ 0.04 |
α2-Globulin | ∼ 0.08 |
β-Globulin | ∼ 0.12 |
γ-Globulin | ∼ 0.16 |
Fibrinogen | ∼ 0.03 |
Albumin is the main plasma protein (3.0–4.5 g/dl; plasma half-life 15–19 days; ∼ 60% of total protein) synthesized byhepatocytes. Newly synthesized albumin is secreted into the circulation at the rate of about 15 g/d in humans. It binds many small molecules for transport in the blood and participates in blood colloidal osmotic pressure (Π), which keeps fluids within the vasculature. Plasma albumin indeed yields about 65% of osmotic pressure, whereas globulins and fibrinogen contribute according to their plasma concentrations. Molecular structure and charge of albumin facilitate the cotransport of numerous hydrophobic molecules, such as enzymes and hormones, across the endothelium (Sect. 9.6). In the interstitial space, it also serves as a major interstitial osmotic agent, hence transendothelial osmotic pressure gradient. Extravasated albumin is recycled into blood circulation by lymphatic vessels. Like IgG, albumin binds the major histocompatibility complex-related Fc receptor at low pH. This interaction shields these proteins from degradation.
Like fibrinogen,globulins induce reversible RBC aggregation in stagnant blood regions.8 Several kinds of globulins exist: (1) α-globulins that transport chemical species, such as thyroxine and retinol (vitamin-A); β-globulins such astransferrin; and γ-globulins, most of the antibodies (0.1–0.4 g/dl of α1-globulins, 0.5–1 g/dl of α2-globulins, 0.7–1.2 g/dl of β-globulins, and 0.5–1.6 g/dl of γ-globulins).
1.3.4 Plasma Lipids and Lipoproteins
The lipoprotein structure shields water-insoluble lipids (cholesteryl esters and triglycerides) from water, as it encapsulates them with polar lipids and proteins. However, core lipids can move between lipoproteins. The 4 main types of circulating lipoproteins that differ in size, density, and content includechylomicrons, andvery-low-density (VLDL),low-density (LDL), andhigh-density (HDL) lipoproteins (Table 1.6, Fig. 1.2).
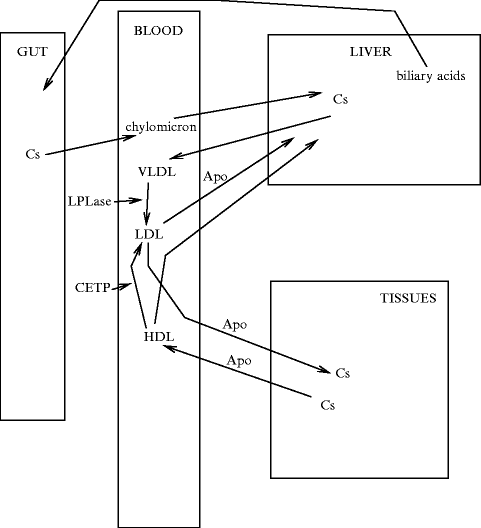
Table 1.6
Size and lipid content (%) of lipoproteins.
Chylomicron | VLDL | LDL | HDL | |
|---|---|---|---|---|
Size (nm) | 80–500 | ∼ 50 | ∼ 20 | ∼ 10 |
Cholesterol | ∼ 0.02 | ∼ 0.07 | ∼ 0.08 | ∼ 0.04 |
Cholesterol esters | ∼ n 0.03 | ∼ 0.12 | ∼ 0.42 | ∼ 0.15 |
Phospholipids | ∼ 0.07 | ∼ 0.18 | ∼ 0.22 | ∼ 0.30 |
Triglycerides | ∼ 0.86 | ∼ 0.55 | ∼ 0.06 | ∼ 0.04 |
Proteins | ∼ 0.02 | ∼ 0.08 | ∼ 0.22 | ∼ 0.47 |

Fig. 1.2
Cholesterol turnover
Lipoproteins convey cholesterol esters (CsE) and triglycerides (TG) in blood.9Triglycerides are delivered to muscles and adipose tissues for energy production and storage (blood TG concentration < 1.2 g/l at 20 years old and < 1.6 g/l at 60 years old).10 Excess lipid and glucid intake leads to conversion into triacylglycerols in the liver that are packaged into VLDLs and released into the circulation. VLDL particles (size 30–80 nm) contain also cholesteryl esters andapolipoproteins (ApoB100, ApoC1–ApoC3, and ApoE).
Intermediate-density lipoproteins (IDL) are formed from VLDLs bylipoprotein lipase after triacylglycerol removal.11 IDL particles become LDLs (size 20 nm) after losing the whole TG content. Plasma LDL contains cholesterol esters in its core and a hydrophilic coat composed of cholesterol, phospholipids, and ApoB100.
Blood lipids require many types of molecules from their synthesis, exocytosis out of producing cells, and blood transport, to cell capture (receptors) and endocytosis, and degradation (Table 1.7).
Table 1.7




Lipids and associated genes (Sources: [4, 5]). Investigations commonly examine high-density and low-density lipoprotein cholesterol, and total plasma triglycerides that are carried mainly in very-low-density lipoproteins and their remnants. Involved genes encode (1) apolipoproteins (ApoA–ApoC and ApoE); (2) lipoprotein receptor (LDLR) and multiligand endocytic receptorsortilin (Sort1) for lipoprotein lipase (LPase) that acts as a sorting receptor in the Golgi body and clearance receptor on the cell surface; (3) transporters of cholesterol (ABCa1) and cholesterol ester (CETP); (4) transcription factor Max-like protein X (MLX)-interacting protein-like (MLXIPL; a.k.a. class-D basic helix–loop–helix protein bHLHd14 and Williams-Beuren syndrome chromosomal region 14 protein [WBSCR14] that activates triglyceride synthesis in a glucose-dependent manner, as it binds to carbohydrate response elements of promoters of triglyceride synthesis genes; (5) enzymes of cholesterol synthesis such as mevalonate kinase (MvK) and degradation such as methylmalonic aciduria B-type protein (MMAB; a.k.a. cob(I)alamin adenosyltransferase); (6) glycosyltransferases, such as β(1,3)-galactosyltransferase 4 (β3GalLT4; a.k.a. 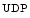 galactose β
galactose β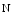 acetyl glucosamine), β(1,3)-galactosyltransferase polypeptide 4), β(1,4)-galactosyltransferase 4 (β4GalLT4), and protein-UDP
acetyl glucosamine), β(1,3)-galactosyltransferase polypeptide 4), β(1,4)-galactosyltransferase 4 (β4GalLT4), and protein-UDP 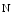 acetyl galactosaminyltransferase 2 (GalNT2; a.k.a. UDP–
acetyl galactosaminyltransferase 2 (GalNT2; a.k.a. UDP–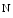 acetyl α
acetyl α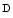 galactosamine–polypeptide
galactosamine–polypeptide 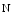 acetylgalactosaminyltransferase 2); (7) lipoprotein (LPase; LPL gene), hepatic (LipC), and endothelial (LipG) lipases; and (8) inhibitor of lipase such as angiopoietin-like protein-3 (AngptL3; a.k.a. angiopoietin-5). Other targets include 3-hydroxy 3-methylglutaryl coenzyme-A reductase (HMGCR); cadherin, EGF-like, LAG-like, and seven-pass receptor CELSR2; cartilage intermediate layer protein CILP2; lysolecithin cholesterol acyltransferase (LCAT); myosin-binding protein H-like molecule (MyBPHL); neurocan (NCan; a.k.a. chondroitin sulfate proteoglycan CSPG3); preB-cell leukemia homeobox transcription factor PBx4; proline/serine-rich coiled-coil protein PSRC1; proprotein convertase subtilisin–kexin-like peptidase PCSK9 (a.k.a. neural apoptosis-regulated convertase); sterol regulatory element-binding protein (SREBP); and tribbles homolog Trib1.
acetylgalactosaminyltransferase 2); (7) lipoprotein (LPase; LPL gene), hepatic (LipC), and endothelial (LipG) lipases; and (8) inhibitor of lipase such as angiopoietin-like protein-3 (AngptL3; a.k.a. angiopoietin-5). Other targets include 3-hydroxy 3-methylglutaryl coenzyme-A reductase (HMGCR); cadherin, EGF-like, LAG-like, and seven-pass receptor CELSR2; cartilage intermediate layer protein CILP2; lysolecithin cholesterol acyltransferase (LCAT); myosin-binding protein H-like molecule (MyBPHL); neurocan (NCan; a.k.a. chondroitin sulfate proteoglycan CSPG3); preB-cell leukemia homeobox transcription factor PBx4; proline/serine-rich coiled-coil protein PSRC1; proprotein convertase subtilisin–kexin-like peptidase PCSK9 (a.k.a. neural apoptosis-regulated convertase); sterol regulatory element-binding protein (SREBP); and tribbles homolog Trib1.
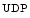 galactose β
galactose β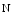 acetyl glucosamine), β(1,3)-galactosyltransferase polypeptide 4), β(1,4)-galactosyltransferase 4 (β4GalLT4), and protein-UDP
acetyl glucosamine), β(1,3)-galactosyltransferase polypeptide 4), β(1,4)-galactosyltransferase 4 (β4GalLT4), and protein-UDP 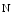 acetyl galactosaminyltransferase 2 (GalNT2; a.k.a. UDP–
acetyl galactosaminyltransferase 2 (GalNT2; a.k.a. UDP–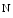 acetyl α
acetyl α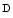 galactosamine–polypeptide
galactosamine–polypeptide 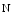 acetylgalactosaminyltransferase 2); (7) lipoprotein (LPase; LPL gene), hepatic (LipC), and endothelial (LipG) lipases; and (8) inhibitor of lipase such as angiopoietin-like protein-3 (AngptL3; a.k.a. angiopoietin-5). Other targets include 3-hydroxy 3-methylglutaryl coenzyme-A reductase (HMGCR); cadherin, EGF-like, LAG-like, and seven-pass receptor CELSR2; cartilage intermediate layer protein CILP2; lysolecithin cholesterol acyltransferase (LCAT); myosin-binding protein H-like molecule (MyBPHL); neurocan (NCan; a.k.a. chondroitin sulfate proteoglycan CSPG3); preB-cell leukemia homeobox transcription factor PBx4; proline/serine-rich coiled-coil protein PSRC1; proprotein convertase subtilisin–kexin-like peptidase PCSK9 (a.k.a. neural apoptosis-regulated convertase); sterol regulatory element-binding protein (SREBP); and tribbles homolog Trib1.
acetylgalactosaminyltransferase 2); (7) lipoprotein (LPase; LPL gene), hepatic (LipC), and endothelial (LipG) lipases; and (8) inhibitor of lipase such as angiopoietin-like protein-3 (AngptL3; a.k.a. angiopoietin-5). Other targets include 3-hydroxy 3-methylglutaryl coenzyme-A reductase (HMGCR); cadherin, EGF-like, LAG-like, and seven-pass receptor CELSR2; cartilage intermediate layer protein CILP2; lysolecithin cholesterol acyltransferase (LCAT); myosin-binding protein H-like molecule (MyBPHL); neurocan (NCan; a.k.a. chondroitin sulfate proteoglycan CSPG3); preB-cell leukemia homeobox transcription factor PBx4; proline/serine-rich coiled-coil protein PSRC1; proprotein convertase subtilisin–kexin-like peptidase PCSK9 (a.k.a. neural apoptosis-regulated convertase); sterol regulatory element-binding protein (SREBP); and tribbles homolog Trib1.Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


