Bivalirudin (BIV) is superior to a heparin/glycoprotein IIb/IIIa receptor inhibitor (GPI) strategy with respect to net adverse cardiovascular events for ST-segment elevation myocardial infarction (STEMI) percutaneous coronary intervention (PCI), albeit with an increased risk of acute stent thrombosis. We hypothesized that a 2-hour BIV infusion after PCI (BIV + 2) could be used without increased bleeding risk as a potential method of mitigating early thrombotic risk. We analyzed a 6-center regional protocol involving routine therapy with aspirin, clopidogrel, and bolus heparin followed by primary PCI for STEMI using BIV. All consecutive patients presenting with STEMI requiring primary PCI were included (2009 to 2011). We compared baseline characteristics and clinical outcomes of the University of Vermont Regional Registry to the historical groups of BIV (BIV terminated at end of PCI) or unfractionated heparin/GPI from the HORIZONS trial and determined independent predictors of bleeding. Of 346 patients undergoing PCI for STEMI, 98% received BIV; 82% of patients received BIV + 2, and 13.3% of all patients receiving BIV received GPI bailout. All-cause mortality was 3.1%. Overall bleeding rates were 50% less than in the HORIZONS GPI arm and similar to the HORIZONS BIV arm. Acute stent thrombosis occurred in <1.0% of patients. Bailout GPI was a potent independent predictor of bleeding complications. In conclusion, BIV + 2 is a feasible regional pharmacologic algorithm for STEMI PCI; BIV + 2 for STEMI PCI is not associated with increased bleeding risk and warrants further study as a mechanism of mitigating very early thrombosis risk.
In the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial, bivalirudin (BIV) was associated with decreased bleeding complications and mortality compared to unfractionated heparin (UFH)/glycoprotein inhibitor (GPI), albeit with an increased risk of acute stent thrombosis. Acute stent thrombosis risk associated with BIV occurs within 1 hour to 6 hours of ST-segment elevation myocardial infarction (STEMI) percutaneous coronary intervention (PCI) and suggests a role for platelet activation soon after PCI. A prolonged BIV infusion that continues anticoagulation until oral platelet inhibition reaches maximal activity may help ameliorate this acute risk. Although extended BIV infusions have been studied in acute coronary syndrome populations, the impact of an extended BIV infusion on bleeding and thrombosis compared to historical controls from the HORIZONS trial has not been evaluated. We previously showed BIV can be incorporated into a regional STEMI strategy using a strategy of stacking full-dose UFH and full-dose BIV. In the present study we hypothesized that a STEMI BIV protocol with 2-hour extension of infusion after PCI (BIV + 2) could be used in a regional STEMI program without increased bleeding risk compared to the HORIZONS BIV protocol (BIV stopped at end of PCI procedure). We also explored the effect of BIV + 2 on the incidence of acute stent thrombosis, focusing on the very early 6-hour window of increased risk found in the HORIZONS trial. We identified predictors of bleeding complications in this regional STEMI BIV registry.
Methods
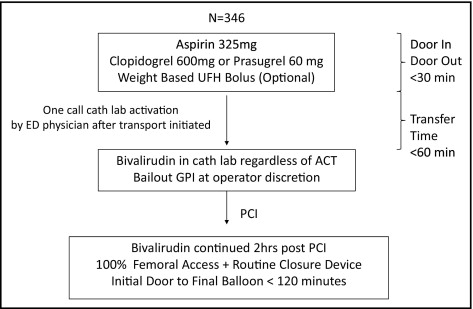
We performed a retrospective analysis of a 6-center regional protocol involving recommended therapy with aspirin, clopidogrel 600 mg, and UFH bolus followed by transfer for primary PCI for STEMI using BIV anticoagulation ( Figure 1 ).
All consecutive patients with primary PCI from January 1, 2009 to June 30, 2011 were included in the analysis including patients presenting with cardiogenic shock and cardiac arrest. Patients presenting with STEMI who did not require PCI owing to coronary angiographic findings (medical therapy or referred for coronary bypass surgery) were excluded from the analysis.
The STEMI network consisted of 5 non-PCI hospitals surrounding the only PCI hospital in Vermont. The algorithm has been previously described and is outlined in Figure 1 : goal emergency room door-in/door-out time at the non-PCI hospitals was <30 minutes; transfer time was <60 minutes; and initial door-to-balloon time goal was <120 minutes for all transferred patients with STEMI. Definition of STEMI included >1-mm STE in ≥2 contiguous leads, new left branch bundle block, or posterior infarction as defined in the HORIZONS trial.
Patients presenting to the 5 non-PCI centers were given clopidogrel 600 mg, aspirin 325 mg, and a weight-adjusted UFH bolus immediately before transfer. Patients presenting to the PCI center were given clopidogrel 600 mg or prasugrel 60 mg and aspirin 325 mg with optional UFH bolus ( Figure 1 ). Single-telephone call catheter laboratory activation by an emergency room physician after transport initiation was mandated. At arrival to the PCI center catheterization laboratory and regardless of activated clotting time, recommended antithrombotic therapy was a bolus of BIV 0.75 mg/kg followed by a 1.75-mg/kg/hour infusion with bailout GPI and thrombectomy at the discretion of the operator. BIV was continued at the established infusion rate for 2 hours after PCI. Interventional procedures were performed exclusively through the femoral approach and vascular closure devices were used immediately at the end of the PCI when the femoral anatomy was amenable to closure; if femoral anatomy was not amenable to closure (i.e., femoral arterial disease), sheaths were pulled 2 hours after completion of BIV infusion.
Demographics, medical histories, and clinical characteristics were reviewed from medical records of all patients presenting with STEMI requiring primary PCI during the study period. Procedural and angiographic data of enrolled patients were abstracted from the catheterization laboratory database. We analyzed complications including major bleeding, postprocedure MI, acute stent thrombosis, and death. Values were presented as mean ± SD. Clinical characteristics and outcomes between the University of Vermont Regional Registry and the HORIZONS BIV arm were compared using Student’s t test for continuous variables and chi-square test for dichotomous variables. Multivariate logistic regression analysis was performed to identify independent predictors of major bleeding complications; clinical variables that were determined to be univariate predictors with a p value <0.10 were entered into the model as covariates. Additional multivariate logistic regression analysis was performed to determine the predictive value of bailout GPI while controlling for independent clinical risk factors for bleeding complications.
The primary end point of the study was incidence of major bleeding during the index hospitalization compared to the historical HORIZONS BIV group. Secondary end points were rates of 6-hour (early acute) and 24-hour (acute) stent thrombosis, postprocedure MI, in-hospital death, and net adverse cardiovascular events. Mortality was defined as all-cause death during the index hospitalization. Net adverse cardiovascular events included death, MI, stent thrombosis, and major bleeding. Major bleeding was defined according to the HORIZONS trial. Acute definite/probable stent thrombosis was defined according to modified Academic Research Consortium criteria.
Results
In total 346 patients from 6 medical centers who presented with STEMI underwent primary PCI during the study period and were included in this registry. Approximately 1/2 of patients presented to the non-PCI referral centers ( Table 1 ) and 1/4 of all patients had previous revascularization with PCI or coronary artery bypass grafting. This was a high-risk group with almost 1/5 of patients having cardiogenic shock or ventricular arrhythmias complicating their presentation. Almost all patients received aspirin (99%) and a loading dose of clopidogrel 300 or 600 mg (97%). Upstream weight-adjusted UFH bolus was given to 60% of patients without any minimum time delay from UFH to subsequent BIV. BIV was stacked on top of the UFH bolus to avoid delays associated with initiating BIV in STEMI referral centers : mean initial door to final balloon time was 95 minutes for all transferred patients with STEMI and consistent with the American College of Cardiology/American Heart Association 2011 guidelines (<120-minute initial door to first balloon time).
| Variable | |
|---|---|
| Men | 74.8 |
| Age (years) | 62.4 ± 12.9 |
| Age >75 years | 17.9% |
| Body surface area (m 2 ) | 1.96 ± 0.32 |
| Weight (kg) | 86.1 ± 21.1 |
| Previous heart failure | 4.1% |
| Peripheral arterial disease | 5.5% |
| Diabetes mellitus | 20.2% |
| Previous percutaneous coronary intervention | 20.5% |
| Hypertension | 60.7% |
| Previous coronary artery bypass surgery | 7.5% |
| Hyperlipidemia | 61.9% |
| Current smoker | 38.7% |
| Renal failure (creatine >1.5 or glomerular filtration rate <60) | 6.9% |
| Former smoker | 47.4% |
| Family history of coronary artery disease | 37.2% |
| Previous stroke | 5.2% |
| Known bleeding disorder | 1.7% |
| Initial presentation | |
| Presentation to percutaneous coronary intervention center | 47.4% |
| Cardiogenic shock | 12.4% |
| Cardiac arrest | 9.0% |
| Acute pulmonary edema | 10.1% |
| Ventricular tachycardia/ventricular fibrillation | 9.8% |
| Initial pharmacology | |
| Unfractionated heparin bolus | 59.8% |
| Aspirin | 99.1% |
| Clopidogrel load | 97.1% |
| Prasugrel | 10.4% |
| Mean unfractionated heparin bolus (U) | 4,964 ± 795 |
| Initial angiographic characteristics | |
| Number of coronary artery involved | |
| 1 | 151 (47.3%) |
| 2 | 100 (31.4%) |
| 3 | 68 (21.3%) |
| Thrombolysis In Myocardial Infarction grade | |
| 0/1 | 255 (81.7%) |
| 2 | 40 (12.8%) |
| 3 | 17 (5.5%) |
Of the entire registry 98% were treated with BIV during STEMI PCI. Overall 82% of patients received the BIV + 2 regimen. The 18% of patients not receiving the BIV + 2 regimen were treated with a BIV infusion stopped after PCI. Clinical characteristics of patients in the University of Vermont Regional Registry were different from those in the HORIZONS BIV arm ( Table 2 ). The registry has a high incidence of cardiogenic shock, out-of-hospital cardiac arrest, and ventricular arrhythmias. Registry patients were also more likely to have diabetes (p = 0.03), previous PCI (p <0.0001), and previous coronary artery bypass grafting (p = 0.0002) compared to HORIZONS BIV patients. Rate of bailout GPI in the regional registry was significantly higher than in the HORIZONS BIV arm (13.3% vs 7.5%, p <0.01). Reasons for discontinuation of BIV before completion of the 2-hour infusion were left to the operator’s discretion. Bailout GPI was much more frequent in patients with BIV stopped at the end of PCI (52% vs 4.6%, p <0001). Use of bailout GPI changed over time: in the third year of the registry, use of bailout GPI became nearly identical (7%) to that of the HORIZONS BIV group ( Figure 2 ).
| Variable | UVM STEMI Registry (n = 346) | HORIZONS BIV Arm (n = 1,800) | p Value |
|---|---|---|---|
| Median age (years) | 62 | 59.8 | — |
| Men | 74.8% | 77.1% | 0.35 |
| Diabetes mellitus | 20.2% | 15.6% | 0.03 |
| Hypertension | 60.7% | 51.8% | 0.002 |
| Hyperlipidemia | 61.9% | 43.4% | <0.0001 |
| Current smoker | 38.7% | 47.2% | 0.0037 |
| Previous percutaneous coronary intervention | 20.5% | 10.5% | <0.0001 |
| Previous coronary artery bypass grafting | 7.5% | 3.3% | 0.0002 |
| Median weight (kg) | 84.1 | 80 | — |
| Renal insufficiency (creatine >1.5 or glomerular filtration rate <60) | 6.9% | 15.8% | <0.0001 |
Major bleeding was 4.3% in the entire STEMI registry and nearly identical to that of the HORIZONS BIV arm major bleeding rate (p = 0.63; Table 3 ). Of the 82% of patients who received BIV + 2, rate of major bleeding was even lower and tended to be less than the HORIZONS BIV arm (2.5 vs 4.9%, p = 0.067). Bleeding rates of the BIV + 2 STEMI registry were 50% less than in the HORIZONS GPI arm (p = 0.01), and for patients actually treated with a 2-hour infusion of BIV, major bleeding was even more significantly decreased compared to the HORIZONS GPI arm (2.5 vs 8.3%, p = 0.0006).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


