Fig. 31.1
Algorithm for decision between left ventricular and biventricular support in patients with terminal heart failure at our institution [8]. S/L axis indicates ratio of short-axis diameter to long-axis diameter. RVEDD right ventricular end-diastolic diameter, RVEF right ventricular ejection fraction, RA right atrium, PVR pulmonary vascular resistance, BVAD biventricular assist device, and LVAD left ventricular assist device
31.4 Operative Strategy
Only patients with extremely diminished RV function who are clear candidates for BVAD support receive implantation of a second HVAD as RVAD primarily. In all other patients, we recommend to implant first one HeartWare HVAD® device as an LVAD, and then to try to wean the patient from the heart-lung machine and to stabilize the hemodynamic situation. If this cannot be achieved without administering excessive catecholamine dosages, a second pump should be implanted into either the right ventricle or right atrium as an RVAD.
Alternatively, after implantation of one system as an LVAD and evaluation of whether the patient needs additional right ventricular support, a cost-effective temporary RVAD system (Levitronix CentriMag, Levitronix GmbH, Zurich, Switzerland) can be implanted. In the few patients who show no postoperative recovery of right ventricular function within a reasonable period of time, the temporary RVAD can later be replaced by a second HVAD system.
31.5 Implantation Procedure
Only in patients with a deep chest can connection of the pump to the free wall of the right ventricle be recommended (◘ Figs. 31.2 and 31.3). In slim patients with a small chest and in all small-framed patients, this would bear a significant risk for compression of the RV during and after chest closure. Therefore, in all small patients, we recommend connection of the pump to the right atrium (◘ Figs. 31.4 and 31.5) or to the inferior (diaphragmatic) wall of the RV [9, 10].
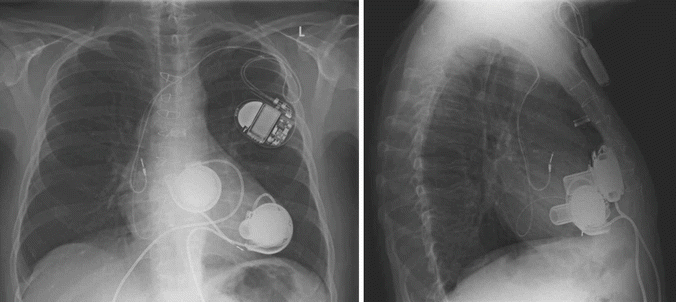
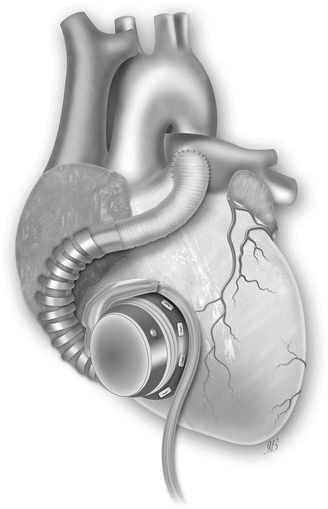

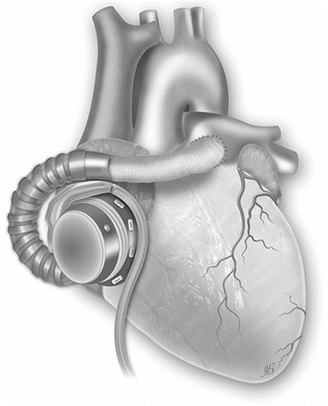

Fig. 31.2
Chest roentgenogram of a patient after implantation of two HeartWare HVAD® ventricular assist devices for biventricular support. The right pump is connected to the anterior free wall of the right ventricle

Fig. 31.3
The HVAD pump sewing ring is affixed with interrupted or running sutures to the anterior free wall of the right ventricle (RV) just below the outflow tract. The inflow cannula is then installed through the sewing ring into the RV. Outflow graft is attached to the main pulmonary artery (Picture by Ilaria Bondi’s Peppermint Advertising)

Fig. 31.4
Chest roentgenogram of a patient after switch of the right HeartWare HVAD® pump from the anterior free wall of the right ventricle to the right atrium

Fig. 31.5
Right ventricular support by cannulating the body of the right atrium (Picture by Ilaria Bondi’s Peppermint Advertising)
To allow for a “physiological” flow range of 3 to 6 liters per minute within a pump speed setting of between 2200 and 3500 rpm, as usually set when the HVAD® is used as an LVAD and as recommended by the manufacturer, the afterload of the right pump should be artificially increased. We reduce the outflow graft diameter to such a degree that the afterload of the RVAD reaches the normal levels of the systemic circulation. This can be achieved by a reduction to an inner graft diameter of approximately 5 mm in patients with normal and to 6–7 mm in patients with elevated pulmonary vascular resistance. Diameter reduction can be performed by side clamping and narrowing the graft with a suture (6×0 Prolene) or by placement of titanium clips. To ensure definite and reproducible reduction of the diameter, Hegar bars are used for calibration. The length of the narrowed section should be about 35 mm as this length influences the added afterload according to the Hagen-Poiseuille equation.
To reduce the effective length of the inflow cannula, we add two 5 mm silicon suture rings covered with Dacron velour (in-house product of the Deutsches Herzzentrum Berlin, made by Berlin Heart GmbH, Berlin, Germany) to the original HVAD® implantation ring. If this is not available, handmade rings of Dacron velour can be tailored (◘ Fig. 31.6). The rings are fixed to the original “apical” fixation ring using BioGlue (CryoLife, Atlanta, GA). These additional rings prevent the cannula from deep penetration into the right ventricular cavity.


Fig. 31.6
Reduction of the effective length of the inflow cannula with two Dacron velour-covered 5 mm silicon suture rings
31.6 Postoperative Patient Management and Anticoagulation
All patients receive the standard postoperative care for VAD patients. Postoperative catecholamine support – if necessary – should consist of noradrenaline only.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


