Biomarker
Study endpoints
Endothelins
Endothelin-1
Hemodynamic measurements, survival
Big ET-1
Hemodynamic measurements, 6MWD
ET-1/ET-3
Hemodynamic measurements, NYHA FC, survival
CT-pro-ET-1
Clinical severity, hospitalizations, survival
vWF
Hemodynamic measurements, survival
Angiopoietin
Hemodynamic measurements, survival
Circulating endothelial cells
Hemodynamic measurements, treatment-response
Interleukins
Survival
Osteopontin
Hemodynamic measurements, 6MWD, NYHA FC, survival
Pentraxin-3
Screening
Adiponectin
N/A
Uric acid
Hemodynamic measurements
Isoprostanes
Survival
BNP/NT-pro-BNP
Hemodynamic measurements, NYHA FC, 6MWD, treatment-response
Troponin
Hemodynamic measurements, survival
Renin-angiotensin-Aldosterone system
Hemodynamic measurements, disease progression
Pim-1
Screening, survival
Markers of renal dysfunction
Survival
Serum sodium
Survival
Hemoglobin A1c
Survival
Biomarkers in PAH can be divided into five major categories: biomarkers of endothelial dysfunction, biomarkers of inflammation and oxidative stress, biomarkers of right ventricular (RV) failure, biomarkers of pulmonary arterial remodeling, and other biomarkers.
Biomarkers of Endothelial Dysfunction
Endothelial dysfunction and eicosanoid imbalance play an integral role in the pathogenesis of PAH. Several markers of endothelial dysfunction have hence been studied as potential biomarkers in PAH.
Endothelins are a family of naturally occurring peptides that include endothelin-1 (ET-1), endothelin-2 (ET-2) and endothelin-3 (ET-3). ET-1 is abundant in the human lung and is the most potent vasoconstrictor, mainly released from the vascular endothelium [5]. ET-1 has been implicated in the pathogenesis of PAH [6, 7]. Elevated plasma ET-1 levels have been detected in patients with PAH [7–10] and in one study ET-1 levels correlated with the right atrial pressure and pulmonary artery oxygen saturation [7], both markers of prognosis. ET-1 levels have also been shown to be inversely correlated with survival of PAH patients on conventional therapy [11]. Another small study of 16 PAH patients showed that the levels of ET-1 and its precursor big ET-1 were strongly correlated with pulmonary vascular resistance (PVR), mean pulmonary artery pressure (mPAP), cardiac output (CO), cardiac index (CI) and 6-min walk distance (6MWD) [12]. In a study of 33 treatment-naïve PAH patients, the ET-1/ET-3 ratio has been shown to be positively correlated with the right atrial pressure and the New York Heart Association functional class (NYHA FC), and negatively correlated with mixed venous oxygen saturation, and associated with survival [13]. More recently, a study of 28 PAH patients evaluated COOH-terminal pro-endothelin-1 (CT-pro-ET-1), which is derived from the ET-1 propeptide in equal amounts as ET-1, as a potential biomarker [14]. The study found that CT-pro-ET-1 plasma levels at baseline were associated with the clinical severity of the disease and 12-months’ hospitalizations due to PAH-worsening, heart/lung transplantation, or all-cause mortality. Despite these associations, endothelins have not been adopted as a valid biomarker in PAH for several reasons. First, ET-1 levels have been shown to be elevated in African ethnicity, old age and male sex and levels are reduced in individuals treated with angiotensin converting enzyme inhibitors, statins, vasodilators, or β-blockers [15]. Second, there are no studies evaluating the utility of endothelins as a screening tool for PAH. Third, in patients treated with endothelin-receptor antagonists, it is difficult to interpret changes in endothelin levels over time as attributable to treatment effect or underlying disease. Finally, endothelins have not been validated in large prospective cohorts.
Plasma von Willebrand factor (vWF) is a large glycoprotein that is produced in endothelial cells and megakaryocytes. vWF plays an important role in clot formation by stabilizing and activating factor VIII and by binding to IIb/IIIa receptors, recruiting and activating platelets. Elevated levels of vWF have been associated with worse outcomes in healthy individuals, as well as patients with congestive heart failure, coronary artery disease and acute respiratory distress syndrome [16–18]. Patients with IPAH have, on average, higher levels of vWF as compared to patients with PAH from other etiologies, such as congenital heart disease patients [19, 20]. Higher vWF levels have been associated with worse survival in patients with idiopathic, familial, anorexigen use associated- and congenital heart associated-PAH [21, 22]. A small study of 10 PAH patients showed that vWF proteolysis paralleled hemodynamic improvements after initiation of prostacyclin therapy [23]. vWF levels were also shown to be predictive of future development of elevated pulmonary artery pressures in patients with limited scleroderma, a condition recognized as a risk factor for PAH development; however, vWF levels decreased over the 3 year follow-up period of the study [24], limiting this marker only as a research tool at this time.
Angiopoietin -1 (Ang-1) and its antagonist angiopoeitin-2 (Ang-2) are angiogenic factors that bind to the tyrosine kinase with immunoglobulin-like and EGF-like domains 1 (Tie) family of receptors and are responsible for vascular development, remodeling and maturation [25]. Imbalances in the angiopoetin-Tie2 receptor system have been implicated in the pathogenesis of IPAH [26, 27]. Plasma levels of Ang-1, Ang-2, soluble Tie2 and vascular endothelial growth factor (VEGF) were elevated in patients with IPAH as compared to healthy controls [28]. Ang-2 but not the other angiogenic markers correlated with CI, PVR, and mixed venous oxygen saturation and changes in Ang-2 after initiation of therapy correlated with mean right atrial pressure, PVR and mixed venous oxygen saturation [28]. Ang-2 was also an independent predictor of mortality in IPAH [28].
Circulating endothelial cells (CECs) are thought to shed in response to endothelial injury. One study found that the CECs count was increased in the serum of patients with IPAH and PAH associated with other etiologies as compared to healthy controls [29, 30]. The CECs count also correlated with hemodynamic parameters in these patients. The number of CECs decreased in response to initiation of PAH-specific therapy in children with PAH [31].
Biomarkers of Inflammation and Oxidative Stress
There is an increasing amount of evidence that implicates inflammation in the pathogenesis of PAH. There is a wide array of inflammatory cytokines that are elevated in patients with PAH, including interleukin-1 beta (IL-1 beta), IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70 and tumor necrosis factor-alpha [32, 33]. IL-6, IL-8, IL-10 and IL-12p70 levels were also shown to predict survival in patients with PAH [33]. Another study demonstrated similar findings with addition of elevated levels of vascular endothelial growth factor (VGEF), platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-beta) and IL-6 [34]. IL-6 was again shown to predict mortality. C-reactive protein (CRP), another inflammatory marker, was found to be elevated in patients with PAH and chronic thromboembolic pulmonary hypertension (CTEPH) when compared with healthy controls, it correlated with survival in patients with PAH and its normalization in response to treatment conferred a survival advantage [35]. In addition, in CTEPH patients, CRP levels decreased after pulmonary endartrectomy [35], suggesting a possible role as a biomarker for assessing the severity and response to therapy in PAH.
Osteopontin (OPN), a multicellular protein, is another cytokine involved in recruitment and retention of macrophages and T cells to sites of inflammation and has also been implicated in the pathogenesis of PAH [36–38]. Plasma OPN levels have been shown to be elevated in patients with idiopathic PAH as compared to healthy controls [39]. OPN levels correlated with hemodynamic parameters such as the right atrial pressure as well as functional parameters, including the 6MWD and NYHA FC. Moreover, baseline OPN levels were predictive of survival in patients with IPAH [39].
Human pentraxin 3 (PTX3) is one of the large proteins in the family of pentraxins, which is synthesized by dendritic cells of myelomonocytic origin in response to proinflammatory signals and Toll-like receptor engagement [40]. PTX3, a protein related to CRP and synthesized by vascular cells, as well as innate immunity cells, regulates cell proliferation, angiogenesis, inflammation, and matrix deposition [41]. In a large study of individuals free of atherosclerotic disease, PTX3 levels correlated with greater RV mass and larger RV end-diastolic volume, independent of common cardiovascular risk factors and left ventricular morphologic changes [42]. PTX3 has been recently investigated in PAH patients. In a case-control study, plasma PTX3 levels were significantly higher in PAH patients as compared to healthy age and sex-matched controls [43]. PTX3 was also shown to perform better than BNP or CRP in patients with PAH associated with connective tissue disease [43].
Adiponectin, a protein released from adipose cells, plays a role in insulin sensitivity as well as influences vascular inflammation, vascular dilation and vascular smooth muscle cell proliferation, all features of PAH pathophysiology [44–47]. In a case-control study, adiponectin levels in PAH patients were elevated compared to controls when matched for age, sex and body mass index [48].
Serum uric acid is a final product of adenine nucleotide degradation in response to tissue ischemia and hypoxia and has been shown to be elevated in several hypoxic conditions including chronic heart failure and chronic obstructive pulmonary disease [49, 50]. Serum uric acid levels were found to be elevated in PAH patients when compared to controls and the elevated uric acid levels were associated with a worse hemodynamic profile as well as increased risk of mortality [51, 52]. Similarly, elevated uric acid levels were correlated with pulmonary artery systolic pressure in patients with pulmonary hypertension (PH) associated with sickle cell disease [53].
15-F2t-isoprostane derives from arachidonic acid metabolism and is a stable product of lipid peroxidation that has been linked with oxidative stress and has been shown to be elevated in both chronic left heart failure and interstitial lung disease. Urinary concentrations and more recently plasma concentrations of 15-F2t-isoprostane were elevated and correlated with poor outcomes in PAH patients [54, 55].
Despite the various associations between inflammatory markers and PAH, none of these biomarkers have been adapted in clinical practice mainly due to lack of validation of their usefulness in diagnosis and prognosis. Most of these associations were derived from observational studies. Serial measurements and longitudinal follow-up are necessary before these investigational biomarkers can be incorporated into clinical practice.
Biomarkers of Right Ventricular Failure
Right ventricular adaptation is a major prognostic factor in PAH and a determinant of mortality. As a consequence, surrogate markers of RV function were investigated for their potential role as predictors of outcome in PAH. Atrial natriuretic peptide (ANP) and BNP are peptide hormones that are produced from cardiomyocytes in response to volume or pressure overload (Fig. 14.1) [56, 57]. ANP secretion is mainly driven by atrial stretch rather than ventricular stretch. On the other hand, BNP is more sensitive to ventricular overload. Natriuretic peptides have been long studied in several forms of heart failure, including RV failure. In addition to its role in assessing left ventricular failure, BNP has been shown to be useful in assessing RV dysfunction. Earlier studies have shown that BNP levels were helpful in differentiating between cardiac and pulmonary causes of dyspnea with elevated levels in patients with chronic obstructive pulmonary disease (COPD) and evidence of cor pulmonale as compared to those with COPD alone [58, 59]. Since then, BNP and NT-pro-BNP have emerged as mainstream biomarkers of RV dysfunction in several forms of PH and in acute pulmonary embolism [60–65]. The first study to demonstrate the prognostic significance of BNP levels examined 60 PAH patients at the time of diagnostic right heart catheterization and on follow-up after treatment with prostacyclin [66]. Patients with a baseline BNP level ≥150 pg/ml or ≥180 pg/ml at follow-up after treatment had significantly worse survival. BNP levels have also been shown to be related to hemodynamic parameters [63–68] and functional impairment [67]. NT-pro-BNP, a byproduct of BNP synthesis, has advantages over BNP, as it is a more stable peptide with a more accurate assay [69]. NT-pro-BNP levels correlated with hemodynamic parameters [70, 71] as well as survival in PAH [72–74]. Nevertheless, both BNP and NT-pro-BNP are affected by several factors, including renal function, weight, age and gender [75–78], which leads to difficulty in determining a single cutoff value. Improvements in NT-pro-BNP have also been shown to correlate with treatment effect in PAH [79, 80]. The significance of serial measurements of NT-pro-BNP has been studied retrospectively in patients with idiopathic PAH [72]. A greater than 15 % decrease per year in NT-pro-BNP level was associated with improved survival. Currently, BNP or its precursor NT-pro-BNP are the only serologic markers recommended to follow and to attempt to normalize in PAH [4].
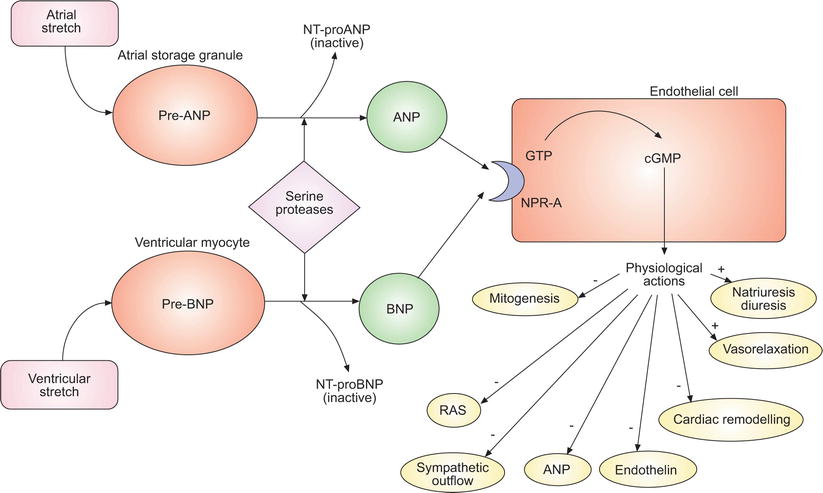

Fig. 14.1
Simplified schematic of the natriuretic peptide system. Natriuretic peptide precursors are released in response to atrial and ventricular stretch, cleaved into active molecules and inactive precursors and convert guanosine 5’-triphosphate (GTP) to cyclic guanosine monophosphate (cGMP), leading to their various physiological actions. ANP atrial natriuretic peptide, NT-proANP N-terminal pro-ANP, NT-proBNP N-terminal ProBNP, NPR-A natriuretic peptide receptor A, RAS rennin-angiotensin system. This material has not been reviewed by European Respiratory Society prior to release; therefore the European Respiratory Society may not be responsible for any errors, omissions or inaccuracies, or for any consequences arising there from, in the content (Reproduced with permission of the European Respiratory Society: Willis et al. [122])
Cardiac troponin T is a biomarker of myocardial damage, which has been used as an indicator of RV dysfunction in patients with PAH and has been shown to correlate with survival in patients with PAH and CTEPH [81]. Levels of troponin T correlated with higher heart rates, lower mixed venous oxygen saturation and higher serum NT-pro-BNP. In this study of 56 patients with PAH or CTEPH, normalization of troponin T levels with treatment was associated with improved survival [81]. In the study from the National PH Registry in Ireland that included 108 PAH patients, BNP and highly sensitive troponin were independent predictors of poor outcome (hazard ratio 6.68 and 5.48, respectively) however a declining 6MWD was found to be a stronger predictor than both (hazard ratio of 12.8) [82]. The main drawback to troponin T is that it is not specific to PAH and can be elevated in patients with left ventricular dysfunction, acute coronary syndrome and patients with acute pulmonary embolism [83].
Biomarkers of Pulmonary Arterial Remodeling
Recently, activation of the renin-angiotensin-aldosterone system (RAAS) has been reported to be a contributor to cardiopulmonary remodeling in PAH similar to systemic cardiovascular diseases [84–89]. In experimental models of PAH, increased plasma and lung tissue levels of angiotensin and aldosterone have been detected and found to correlate with cardiopulmonary hemodynamics and pulmonary vascular remodeling [85, 86]. Moreover, in a cohort of 58 patients with idiopathic PAH, serum renin and angiotensin I levels were increased above the upper limit of normal in the majority of patients, whereas angiotensin II levels were elevated only in a smaller cohort [84]. With follow-up, persistently elevated levels of renin-angiotensin activity were associated with PAH disease progression assessed as a greater than 10 % decrease in the 6MWD and an increased risk of lung transplantation or death [84].
The terminal component of the RAAS, the mineralocorticoid receptor (MR), is directly activated by aldosterone. In a pilot study of patients with unexplained dyspnea, the plasma aldosterone level was increased in patients with PAH, compared with control subjects. Furthermore, aldosterone levels correlated positively with PVR and inversely with CO in a subset of patients with PAH who had severe disease [90]. We and others have shown that MR plays a direct role in the remodeling process of the pulmonary vasculature in experimental pulmonary hypertension and that MR inhibition by MR antagonists attenuates pulmonary hypertension in several experimental models [91, 92].
Collectively, these reports implicate RAAS and MR activation in PAH and suggest that renin, angiotensin and aldosterone levels have the potential to become biomarkers of disease severity.
The pathophysiology of PAH involves pulmonary artery smooth muscle cell (PASMC) proliferation and resistance to apoptosis. A tissue-specific activation of provirus integration site for Moloney murine leukemia virus (Pim-1), a proto-oncogene minimally expressed in healthy cells, was shown to contribute to the activation of NFAT/STAT3 signaling pathway responsible for the sustainability of this phenotype [93]. In a recent study by Renard and colleagues, Pim-1 levels were elevated in patients with IPAH (including a group with vasoreactive phenotype), PAH associated with connective tissue disease, and PAH associated with congenital heart disease, versus controls [94]. Pim-1 levels discriminated effectively between the presence vs. absence of PAH and remained an independent predictor of mortality after adjustment for hemodynamic and biochemical variables [94]. Serum Pim-1 level has, therefore, emerged as a promising biomarker of pulmonary vascular remodeling although these findings need to be replicated in larger trials.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


