(1)
University of Ottawa The Ottawa Hospital, Ottawa, ON, Canada
This chapter tells you
Which beta-blocker is best for your patients.
The pharmacodynamic reasons why atenolol is a relatively ineffective beta-blocker and why the vast worldwide use of atenolol should be curtailed.
More about the important indication for heart failure (HF), for New York Heart Association (NYHA) class II and III and compensated class IV, and for all with left ventricular (LV) dysfunction regardless of functional class; thus, in class I patients with an ejection fraction (EF) <40 % and in those with myocardial infarction (MI) with HF or LV dysfunction without HF, beta-blockers are recommended at the same level as angiotensin-converting enzyme (ACE) inhibitors. Beta-blockers are the mainstay of therapy for heart failure. They decrease total mortality, an effect only modestly provided by ACE inhibitors, and marginally by angiotensin receptor blockers [ARBS]. See discussion under ARBs.
Why beta-blockers should be recommended for diabetic patients with hypertension with or without proteinuria and for diabetic patients with coronary heart disease (CHD). From about 1990 to 2007, most internists proclaimed in editorials and to trainees that these agents were a poor choice in this setting.
More recently, their use as initial agents for the treatment of primary hypertension has been criticized, particularly for diabetics with hypertension; do beta-blocking drugs cause diabetes or is the condition observed, simply, benign glucose intolerance in some? (see Chap. 2)
Why is it incorrect to say that beta-blockers are not advisable for hypertensive patients over age 70, as many teachers, textbooks, and editorials state.
The results of randomized clinical trials (RCTs) that prove the lifesaving properties of these agents.
All their indications.
Salient points that relate to each beta-blocker and show the subtle and important differences confirming that beta-blockers are not all alike. Beta-blockade holds the key, but lipophilic vs. hydrophilic features may be important, and brain concentration may enhance cardioprotection.
Beta-Blockers and Cardioprotection
Sufficient attention has not been paid by the medical profession and researchers regarding the subtle differences that exist amongst the available beta-blocking drugs (Khan 2005). The subtle differences in beta-blockers may provide the solution for the apparent lack of protection of some beta-blockers (Khan and Topol 1996).
The common threads for enhanced cardioprotection are beta1, beta2, and lipophilicity that augment brain concentration and may protect from sudden death. In the timolol study, there was a 67 % reduction in sudden death (The Norwegian Multicenter Study Group 1981).
Timolol is non-cardioselective and lipophilic. No other cardiovascular agent has produced such an outstanding reduction in cardiac sudden deaths, yet the drug is rarely prescribed worldwide.
Propranolol, a beta1, beta2 lipophilic drug, caused a 56 % decrease in early morning sudden death from acute myocardial infarction [MI]; see later discussion of BHAT (1982), and Peters et al. (1989)
Atenolol, a non-lipophilic agent, has been the most prescribed beta-blocker in the USA and worldwide from 1980 to 2007 with more than 44 million prescriptions in the USA annually. The drug is a poorly effective beta-blocker and its use should become obsolete. Unfortunately, investigators and trialists not noticing the subtle differences that exist among the beta-blocking drugs have used atenolol in the majority of large RCTs of hypertension conducted from 1980 to the present time (see Beta-Blocker Hypertension Controversy). Lindholm et al. (2005) from a meta-analysis of hypertension RCTs without regard for beta-blockers’ subtle differences described above reached a conclusion, which was printed on the front cover of the Lancet: “beta-blockers should not remain first choice in the treatment of primary hypertension” (Lindholm et al. 2005). Atenolol was the beta-blocker used in the majority of RCTs analyzed by Lindholm et al.
A rebuttal stated, “by lumping together all randomized hypertension trials involving beta-blockers, Lars Lindholm and colleagues have arrived at misleading conclusions” (Cruickshank 2000). But rebuttals are observed by few clinicians. many notable physicians have endorsed the findings of Lindholm and colleagues and the misleading information has been disseminated worldwide.
Lipophilicity allows a high concentration of drug in the brain. This appears to block sympathetic discharge in the hypothalamus and elevate central vagal tone to a greater extent than water-soluble, hydrophilic agents (Pitt 1992). This may relate to the prevention of sudden cardiac death. Highly lipid-soluble, lipophilic beta-blockers—carvedilol, propranolol, nebivolol, timolol, and metoprolol—reach high concentrations in the brain and are metabolized in the liver.
Atenolol, nadolol, and sotalol are lipid insoluble, show poor brain concentration, and are not hepatic metabolized; they are water soluble, are excreted by the kidneys, and have a long half-life. Pindolol and timolol are about 50 % metabolized and about 50 % excreted by the kidney. Importantly, brain:plasma ratios are ~15:1 for propranolol and timolol, 3:1 for metoprolol, and 1:8 for atenolol.
Lipid-soluble beta-blocking agents with high brain concentration block sympathetic discharge in the hypothalamus better than water-soluble agents (Pitt 1992) and they are more effective in the prevention of cardiac deaths. Bisoprolol is 50 % lipophilic and liver metabolized but does not involve the cytochrome P-450 3A4 pathway. Nebivolol is highly lipophilic. Propranolol and metoprolol have high first-pass liver metabolism. Acebutolol is metabolized to an active metabolite diacetolol, which is water soluble and is excreted by the kidneys. Atenolol, nadolol, and sotalol are not metabolized in the liver. First-pass metabolism varies greatly among patients and can alter the dose of drug required, especially with propranolol.
Cigarette smoking interferes with drug metabolism in the liver and reduces the efficacy of propranolol, other hepatically metabolized beta-blockers, and calcium antagonists (Deanfield et al. 1984).
Beta-blockers are now recommended and used by virtually all cardiologists because they are necessary for the management of acute and chronic ischemic syndromes, manifestations of CHD.
Many internists and family physicians, however, remain reluctant to prescribe beta-blockers in many cardiovascular situations including HF, in hypertension in patients aged >65 years, and in diabetic patients.
Fears that beta-blockers influence lipid levels unfavorably are unfounded. Beta-blocking drugs do not alter low-density lipoprotein (LDL) levels; they may cause a mild increase in levels of triglycerides and may produce a 1 % to ~ 6 % lowering of high-density lipoprotein cholesterol (HDL-C) in fewer than 10 % of patients treated (3). The alteration in HDL-C levels is of minimal clinical concern because the effect is so small, if it occurs at all (Frishman 1997). The clinical importance of this mild disturbance in lipid levels is of questionable significance and should not submerge the prolongation of life and other salutary effects obtained with the administration of beta-blocking drugs (Fig. 1-1).


Fig. 1-1.
Salutary effects of beta-adrenergic blockade.
Since their original discovery by Sir James Black at Imperial Chemical Industries in the UK (Black et al. 1964) and the introduction of the prototype, propranolol, for the treatment of hypertension in 1964 by Prichard and Gillam (1964), more than 12 beta-blocking drugs have become available.
The first edition of Cardiac Drug Therapy in 1984 included a table entitled “Beta-blockers: first-line oral drug treatment in angina pectoris” (Table 1-1); this table indicated the superiority of beta-blockers over calcium antagonists and nitrates. Calcium antagonists were down rated because they decreased blood flow to subendocardial areas; in addition, in the condition for which they were developed, coronary artery spasm (CAS), they were not shown to decrease mortality. This table has never been altered. The 1990s have shown the possible adverse effects and potential dangers of dihydropyridine calcium antagonists. Dihydropyridines increase the risk of death in patients with unstable angina; these agents are not approved for use in unstable angina in the absence of beta-blockade.
Table 1-1
Beta-blockers: first-line oral drug treatment in angina pectoris
Effect on | Beta-blocker | Calcium antagonist | Oral nitrate |
|---|---|---|---|
Heart rate | ↓ | ↑↓ | ↓ |
Diastolic filling of coronary arteries | ↑ | – | – |
Blood pressure | ↓↓ | ↓↓ | – |
Rate pressure product (RPP) | ↓ | – a | – |
Relief of angina | Yes | Yes | Variable |
Blood flow (subendocardial ischemic area)b | ↑ | ↓ | Variable |
First-line treatment for angina pectoris | Yes | No | No |
Prevention of recurrent ventricular fibrillation | Proven | No | No |
Prevention of cardiac death | Proven | No | No |
Prevention of pain owing to CAS | No | Yes | Variable |
Prevention of death in patient with CAS | No | No | No |
The cardiovascular indications for beta-blockers are given in Table 1-2 and allow the author to proclaim that beta-blockers are the cornerstone of cardiac drug therapy.
Table 1-2
Cardiovascular indications for beta-blockers
1. | Ischemic heart disease Stable angina Unstable angina Acute Ml Ml, long-term prevention Silent ischemia |
2. | Arrhythmias VPBs AVNRT Atrial fibrillation Nonsustained VT VT Recurrent VF |
3. | Hypertension Isolated With IHD, diabetes*, LVH, dyslipidemiaa With arrhythmias Perioperative and on intubation Severe, urgent Pheochromocytoma (on alpha-blocker) |
4. | Heart failure: |
5. | Prolonged QT syndrome |
6. | Aortic dissection |
7. | Valvular heart disease Mitral stenosis? tachycardia in pregnancy* Mitral valve prolapse Mitral regurgitation |
8. | To decrease perioperative mortality |
9. | Cardiomyopathy Hypertrophic Dilated |
10. | Marfan’s syndrome |
11. | Neurocardiogenic syncope |
12. | Tetralogy of Fallot |
13. | Aneurysm |
14. | For coronary CT angiogram |
Beta-Receptors
The beta-receptors are subdivided into
The beta1-receptors, present mainly in the heart, intestine, renin-secreting tissues of the kidney, those parts of the eye responsible for the production of aqueous humor, adipose tissue, and, to a limited degree, bronchial tissue.
The beta2-receptors, predominating in bronchial and vascular smooth muscle, gastrointestinal tract, the uterus, insulin-secreting tissue of the pancreas, and, to a limited degree, the heart and large coronary arteries. Metabolic receptors are usually beta2. In addition, it should be noted that
(a)
None of these tissues contains exclusively one subgroup of receptors.
(b)
The beta-receptor population is not static, and beta-blockers appear to increase the number of receptors during long-term therapy. The number of cardiac beta2-receptors increases after beta1-blockade (Kaumann 1991).
(c)
The population density of receptors decreases with age
The beta-receptors are situated on the cell membrane and are believed to be a part of the adenyl cyclase system. An agonist acting on its receptor site activates adenyl cyclase to produce cyclic adenosine-5′-monophosphate, which is believed to be the intracellular messenger of beta-stimulation.
The heart contains beta1– and beta2-adrenergic receptors in the proportion 70:30. Normally, cardiac beta1-adrenergic receptors appear to regulate heart rate and/or myocardial contractility, but in situations of stress, with the provocation of epinephrine release, stimulation of cardiac beta2-receptors may contribute to additional increases in heart rate and/or contractility (Motomura et al. 1990). In HF, cardiac beta1– but not beta2-adrenergic receptors are reduced in number and population, and the myocardium may be less responsive to beta1-inotropic agents.
Mechanism of Action
By definition, beta-blockers block beta-receptors. Structurally, they resemble the catecholamines. Beta-blockers are competitive inhibitors, their action depending on the ratio of beta-blocker concentration to catecholamine concentration at beta-adrenoceptor sites.
Blockade of cardiac beta1-receptors causes a decrease in heart rate, myocardial contractility, and velocity of cardiac contraction. The heart rate multiplied by the systolic blood pressure (i.e., the rate pressure product [RPP]) is reduced at rest and during exercise, and this action is reflected in a reduced myocardial oxygen demand (which is an important effect in the control of angina).
The main in vitro antiarrhythmic effect of beta-blockers is the depression of phase 4 diastolic depolarization. Beta-blockers are effective in abolishing arrhythmias produced by increased catecholamines. Maximum impulse traffic through the atrioventricular (AV) node is reduced, and the rate of conduction is slowed. Paroxysmal supraventricular tachycardia (PSVT) caused by AV nodal reentry is often abolished by beta-blockers, which also slow the ventricular rate in atrial flutter and atrial fibrillation. There is a variable effect on ventricular arrhythmias, which may be abolished if induced by increased sympathetic activity, as is often seen in myocardial ischemia.
Beta-blockers reduce the activity of the renin–angiotensin system by reducing renin release from the juxtaglomerular cells. Also, beta-blockade augments atrial and brain natriuretic peptide (see next section and Suggested Reading).
Beta-blockers interfere with sympathetic vasoconstrictor nerve activity; this action is partly responsible for their antihypertensive effect. Cardiac output usually falls and remains slightly lower than normal with administration of non-intrinsic sympathomimetic activity (ISA) agents. Systemic vascular resistance increases acutely but falls to near normal with long-term administration (Man in’t Veld et al. 1988).
Other Important Clinically Beneficial Mechanisms
Beta-Blockers
Lower plasma endothelin-1 levels, as shown for carvedilol (Krum et al. 1996), and inhibit catecholamine-induced cardiac necrosis (apoptosis) (Cruickshank et al. 1987).
Stimulate the endothelial-arginine/nitric oxide pathway, as shown for the interesting vasodilatory beta-blocker nebivolol (Cockcroft et al. 1995).
Augment atrial and brain natriuretic peptide, upregulate cardiac beta1-receptors, and inhibit stimulatory anti-beta1-receptor autoantibodies.
Beta-Blocker Effect on Calcium Availability
The slow channels represent two of the mechanisms by which calcium gains entry into the myocardial cell. At least two channels exist (Braunwald 1982), namely,
A voltage-dependent channel blocked by calcium antagonists (see Chap. 8).
A receptor-operated channel blocked by beta-receptor blockers that therefore decrease calcium availability inside the myocardial cell. The negative inotropic effect of beta-blockers is probably based on this effect.
Dosage Considerations
The beta-blocking effect is manifest as a blockade of tachycardia when induced by exercise or isoproterenol. The therapeutic response to beta-blockers does not correlate in a linear fashion with the oral dose or plasma level. Differences in the degree of absorption and variation in hepatic metabolism give rise to unpredictable plasma levels, but in addition the same blood level may elicit a different cardiovascular response in patients, depending on the individual’s sympathetic and vagal tone and the population of beta-receptors.
The dose of beta-blocker is titrated to achieve control of angina, hypertension, or arrhythmia. The dose is usually adjusted to achieve a heart rate of 50–60 per min and an exercise heart rate <110 per min. The dosage of propranolol varies considerably (120–480 mg daily) because of the marked but variable first-pass hepatic metabolism. There is a 20-fold variation in plasma level from a given dose of this drug. The proven cardioprotective (CP) dose may be different from the dose necessary to achieve control of angina or hypertension. The effective CP dose (i.e., the dose shown to prevent cardiac deaths in the post-MI patient) for timolol is 10–20 mg daily (Norwegian study 1981), and for propranolol it is within the range 160–240 mg daily. When possible, the dosage of beta-blocker should be kept within the CP range. Other experts are in agreement with this concern for use of the CP dose when possible (Pratt and Roberts 1983).
An increase in the dose beyond the CP dosage (e.g., timolol >30 mg or propranolol >240 mg daily), for better control of angina, hypertension, or arrhythmia, may have a poor reward, that is, there could be an increase in side effects, especially dyspnea, HF, and distressing fatigue.
A review of the clinical literature reveals that too large a dose of beta-blockers may be not only nonprotective but also positively harmful, and this is supported by studies on animals (18). In some patients, one should be satisfied with 75 % control of symptoms and, if necessary, the addition of a further therapeutic agent. The patient is not fearful of anginal pain or high blood pressure—what the patient fears is death. Beta-blockers do prevent cardiac deaths, but they have been shown to do so only at certain doses. In addition, beta-blockers are not all alike; subtle differences can be of importance. Only bisoprolol, carvedilol, metoprolol, propranolol, and timolol have been shown to prolong life in RCTs (see Table 1-3).
Patients may require different drug concentrations to achieve adequate beta-blockade because of different levels of sympathetic tone (circulating catecholamines and active beta-adrenoceptor binding sites). However, plasma levels do not indicate active metabolites, and the effect of the drug may last longer than is suggested by the half-life.
Propranolol may take 4–6 weeks to achieve stable plasma levels because of the extensive hepatic metabolism, but timolol and pindolol undergo less than 60 % metabolism, and constant plasma concentrations are more readily achieved. Therefore, propranolol should be given three times daily for about 6 weeks and then twice daily, or propranolol long-acting (LA) 160–240 mg once daily.
Atenolol, nadolol, and sotalol are excreted virtually unchanged by the kidneys and require alteration of the dosage in severe renal dysfunction, as follows:
(a)
Creatinine clearance of 30–50 mL/min, half the average dose per 24 h.
(b)
Creatinine clearance less than 30 mL/min, half the usual dose every 48 h.
Table 1-3
Beta-blockers: randomized controlled trials showing significant reduction in mortality rate
Mortality | Relative risk reduction | ||||
|---|---|---|---|---|---|
Trial | Drug | Placebo | Drug | % | P |
APSIa | Acebutolol | 34/309 | 17/298 | 48 | 0.019 |
11.0 % | 5.7 % | ||||
BHATb | Propranolol | 188/1,921 | 138/1,916 | 26.5 | <0.01 |
9.8 % | 7.2 % | ||||
Norwegian Post-infarction Studyc | Timolol | 152/939 | 98/945 | 35.8 | <0.001 |
16.2 % | 10.4 % | ||||
Salathia | Metoprolol | 43/364 | 27/391 | 41.5 | <0.05 |
11.8 % | 6.9 % | ||||
Hjalmarson et al. (1981) | Metoprolol | 62/679 | 40/698 | 36.0 | <0.03 |
Post-Ml | 8.9 % | 5.7 % | |||
COPERNICUS | |||||
CAPRICORN | |||||
CIBIS-II | |||||
MERIT-HF | |||||
The oral doses of commonly used beta-blockers are given in Table 1-4. The intravenous (IV) doses are as follows:
Esmolol: 3–6 mg over 1 min, then 1–5 mg/min.
Propranolol: up to 1 mg, at a rate of 0.5 mg/min, repeated if necessary at 2–5-min intervals to a maximum of 5 mg (rarely 10 mg): 0.1 mg/kg.
Metoprolol: up to 5 mg, at a rate of 1 mg/min, repeated if necessary at 5-min intervals to a maximum of 10 mg (rarely 15 mg).
Atenolol: up to 2.5 mg, at a rate of 1 mg/min, repeated if necessary at 5-min intervals to a maximum of 10 mg.
By IV infusion (atenolol): 150 mg/kg over 20 min repeated every 12 h if required.
Table 1-4
Dosage of commonly used beta-blockers
Beta-blocker | Daily starting dose (mg) | Maintenance dose (mg) | Maximum suggested dose (mg) |
|---|---|---|---|
Bisoprolol | 5 | 5–10 | 15 |
Carvedilol | 12.5 | 25–50 | 50 |
Metoprolol | 50–100 | 100–200 | 300 |
Nadolol | 20–80 | 20–160 | 160 |
Propranolol | 40–120 | 40–240 | 320 |
Sotalol | 80–160 | 60–320 | 320 |
Timolol | 5–10 | 20–30 | 30 |
Pharmacologic Properties and Clinical Implications
A clinically useful classification of beta-blockers is given in Fig. 1-2, and their pharmacologic properties are summarized in Table 1-5.
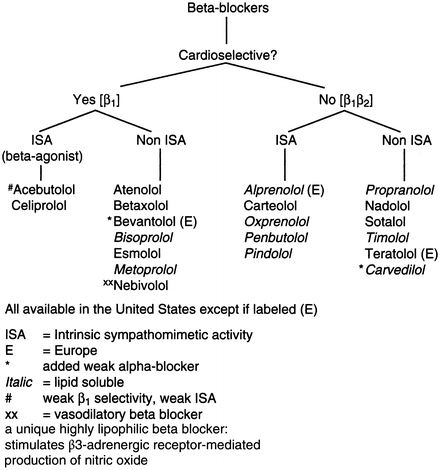

Fig. 1-2.
Classification of beta-blockers.
Table 1-5
Pharmacologic properties of beta-adrenoceptor blockers
Beta-blocker | Propranolol | Timolol | Metoprolol | Nadolol | Atenolol | Carvedilol | Bisoprolol | Acebutolol | Sotalol |
|---|---|---|---|---|---|---|---|---|---|
Equivalent dose (mg) | 80 | 10 | 100 | 60 | 50 | 12.5 | 10 | 400 | 80 |
Potency ratio | 1 | 6–8 | 1 | 1–1.5 | 1–2 | ? | 0.3 | 0.5–1 | |
Relative | Yes | Yes | Yes | No | |||||
Cardioselectivity | No | No | Moderate | No | Strong | No | Strong | Mild | |
Partial agonist activity (ISA) | 0 | 0 | 0 | 0 | 0 | 0 | Nil | Mild | 0 |
Half-life(h) | 2–6 | 2–6 | 2–6 | 14–24 | 7–20 | 14 | 2–6 | 7–20 | |
Variation in plasma level | 20-fold | Sevenfold | Sevenfold | Sevenfold | Fourfold | ? | Fourfold | ||
Lipid solubility | Strong | Moderate | Strong | Nil | Nil | Moderate | Moderate | Weak | Nil |
Absorption (%) | 90 | 90 | 95 | 30 | 50 | 75 | 90 | ||
Bioavailability (%) | 30 | 75 | 50 | 30 | 50 | 40 | 90 | ||
Hepatic metabolism (HM) | HM | 60 % HM | HM | No | No | HM | 50 % | HM | No |
Renal excretion (RE) | 40 % RE | RE | RE | 50 % RE | 60 % RE | RE |
Cardioselectivity
Cardioselectivity implies that the drug blocks chiefly the beta1-receptors and therefore partially spares beta2-receptors in the lungs and blood vessels. A small quantity of beta1-receptors is present in the lungs. Large doses of all beta-blocking drugs block beta2-receptors. Selectivity holds only for small doses and may be lost at the doses necessary for the relief of angina or for the control of hypertension. Atenolol, betaxolol, bisoprolol, metoprolol, bevantolol, esmolol, and, to a lesser degree, acebutolol have less of a blocking effect on beta2-receptors in the lungs, so they are not cardiospecific. They can precipitate bronchospasm in susceptible individuals. Nebivolol is the most beta1-selective, followed by bisoprolol, which is highly selective; the others are moderately to weakly selective.
1.
Bronchospasm. Cardioselective agents may precipitate bronchospasm in a susceptible patient, and this is no different from that of nonselective drugs, except when bronchospasm occurs the patient will respond to a beta 2 -stimulant such as albuterol (salbutamol) if a cardioselective drug was administered. When bronchospasm occurs with the use of nonselective drugs, including pindolol, the spasm may be more resistant to beta-stimulants. Beta-blockers should not be given to patients with bronchial asthma or severe chronic bronchitis or emphysema. It is wise in such patients to choose alternative therapeutic agents. Mild chronic bronchitis is indicated by the following:
Forced expiratory volume greater than 1.5 L.
No hospital emergency room or office treatments for bronchospastic disease.
If a patient with mild chronic bronchitis requires treatment with a beta-blocker for angina, treatment should begin with bisoprolol or metoprolol. If bronchospasm occurs, albuterol (salbutamol) should be added, or the beta-blocker should be discontinued. Bisoprolol is the most cardioselective beta-blocker available and is safer than metoprolol for patients with chronic obstructive pulmonary disease (COPD). In humans, the drug has a twofold higher beta1-selectivity than atenolol.
2.
Peripheral vascular disease (PVD). If a beta-blocker is necessary in a patient with PVD, some clinical trials indicate that it is safer to use a cardioselective drug, atenolol or metoprolol; agents such as carvedilol or bucindolol that cause vasodilation may have a role. Analysis of 11 randomized trials of beta-blockers in patients with PVD showed no worsening of intermittent claudication. Patients with PVD are at high risk for CHD events, and beta-blockers are recommended for all indications. In the United Kingdom Prospective Diabetes Study Group (UKPDS) (19), atenolol did not worsen PVD, and there was a nonsignificant 48 % excess of amputations in the captopril group.
3.
Hypoglycemia stimulates an increase in catecholamine release, which increases blood glucose. The recovery from hypoglycemia may be delayed by nonselective beta-blockers. The incidence of hypoglycemia is higher in insulin-dependent diabetic patients treated with nonselective beta-blockers, whereas both selective and nonselective varieties modify the symptoms of hypoglycemia (with the exception of sweating). Glycolysis and lipolysis in skeletal muscles are mediated mainly by beta2-receptors. Hypoglycemia induced by exercise is more likely to occur with a nonselective beta-blocker. However, evidence to support a greater benefit of selective beta-blockers in joggers is lacking (Breckenridge 1982). Insulin secretion is probably beta2-mediated. Glucose-sulfonylurea-stimulated insulin secretion is inhibited by beta-blockers. Beta-blockers may increase blood glucose by 1.0–1.5 mmol/L, but this glucose intolerance is not type 2 diabetes.
The following points deserve consideration:
Catecholamine stimulation of beta2-receptors produces transient hypokalemia. Thus, cardioselective drugs that spare beta2-receptors may fail to maintain constancy of serum potassium in response to increase in epinephrine and norepinephrine during acute MI (Johansson 1986).
Non-cardioselective agents are superior to selective agents in preventing fluctuations of serum potassium concentration during stress and possibly during acute MI.
Nonselective drugs should confer a greater degree of cardioprotection; carvedilol, propranolol, and timolol have been shown to prevent total mortality and cardiac death in well-controlled RCTs.
Intrinsic Sympathomimetic Activity
Intrinsic Sympathomimetic Activity (ISA) indicates partial agonist activity, the primary agonists being epinephrine and isoproterenol. Beta-blockers that cause a small agonist response (i.e., stimulate as well as block the beta-receptors) include pindolol, alprenolol, acebutolol, celiprolol, carteolol, oxprenolol, and practolol. The last drug has been removed from medical practice because it produced the oculomucocutaneous syndrome. Beta-blockers with ISA cause a slightly lower incidence of bradycardia compared with non-ISA drugs. In practice, this is a minor advantage in the choice of a beta-blocker. The heart rate at rest may be only slightly lowered or unchanged; in patients with angina, a slower heart rate is conducive to less pain on activity.
The RPP at rest is not significantly reduced. Myocardial oxygen consumption is therefore not usually reduced at rest by ISA beta-blockers. Beta-blockers with ISA, therefore, carry no advantage in angina at rest or in angina occurring at low exercise levels; in particular, they do not have a beneficial effect on cardioprotection. The ISA of beta-blockers produces adverse effects on ventricular fibrillation (VF) threshold (Raeder et al. 1983). Acebutolol with weak ISA, however, has been shown to prevent cardiac death. Because they limit exercise tachycardia, these drugs do have a minor role to play in the treatment of patients who have a relatively low resting heart rate (50–60 per min) and in whom further bradycardia may not be acceptable. Even in this subgroup, it is still important to exclude patients with sick sinus syndrome because all beta-blockers are contraindicated here. Renin secretion may remain unaltered or may even be increased by agents with ISA. There may be added sodium and water retention, causing edema. There is no clear-cut evidence that peripheral vascular complications are less frequent when beta-blockers with partial agonist activity are used. Agents with ISA, except acebutolol (very weak ISA), are not recommended by the author because of the aforementioned points; these are not CP drugs.
Membrane-Stabilizing Activity
The quinidine-like or local anesthetic action, membrane-stabilizing activity (MSA), is of no clinical importance, except perhaps for its effect on platelets and in the treatment of glaucoma. It is not related to the antiarrhythmic, antianginal, or CP properties of beta-blockers. Unlike most available beta-blockers, timolol and betaxolol have no MSA. Because of high potency and lack of anesthetic effect, these drugs are the only beta-blockers that have been proved safe and effective in the treatment of glaucoma when used topically.
MSA appears to be important in the management of thyrotoxic crisis, and propranolol has been shown to be more effective than nadolol in this condition.
Effects on Renin
Renin release from the juxtaglomerular cells is suppressed by beta1-receptor blockade; this results in reduced activity of the renin–angiotensin system. Beta-blockers enhance the lifesaving effects of ACE inhibitors in patients with HF or MI (Pitt 1998).
Lipid Solubility
Highly lipid-soluble, lipophilic beta-blockers—carvedilol, propranolol, timolol, and metoprolol—reach high concentrations in the brain and are metabolized in the liver.
Atenolol, nadolol, and sotalol are lipid insoluble, show poor brain concentration, and are not metabolized by the liver; they are water soluble, are excreted by the kidneys, and have a long half-life. Pindolol and timolol are about 50 % metabolized and about 50 % excreted by the kidney.
Brain:plasma ratios are 15:1 for propranolol, 3:1 for metoprolol, and 1:8 for atenolol.
Lipid-insoluble, hydrophilic beta-blockers appear to have a lower incidence of central nervous system (CNS) side effects such as vivid dreams, significant effects on sleep (Kostis and Rosen 1987), impairment of very fast mental reactions (Engler et al. 1986), depression, fatigue, and impotence. Depending on dosage, even the lipid-insoluble drugs can achieve sufficient brain concentration to impair very fast mental reactions. There is little doubt, however, that atenolol causes fewer central side effects than propranolol (Engler et al. 1986). Timolol has been shown to cause less bizarre dreams than pindolol or propranolol in small groups of patients.
Lipid-soluble beta-blocking agents with high brain concentrations block sympathetic discharge in the hypothalamus better than water-soluble agents (Pitt 1992 ) , and they are more effective in the prevention of cardiac deaths.
Bisoprolol is 50 % lipophilic and liver metabolized but does not involve the cytochrome P-450 3A4 pathway; renal elimination is ~50 %.
Plasma Volume
A reduction in cardiac output is usually followed by an increase in plasma volume. Beta-blockers cause a reduction in plasma volume; the exact reason for this is unknown. Pindolol (ISA) may increase plasma volume.
Hepatic Metabolism
Propranolol, oxprenolol, and metoprolol have high first-pass liver metabolism. Timolol and acebutolol have modest lipid solubility and undergo major hepatic metabolism. Acebutolol is metabolized to an active metabolite, diacetolol, which is water soluble and is excreted by the kidneys. Atenolol, nadolol, and sotalol are not metabolized in the liver. First-pass metabolism varies greatly among patients and can alter the dose of drug required, especially with propranolol. Cigarette smoking interferes with drug metabolism in the liver and reduces the efficacy of propranolol, other hepatically metabolized beta-blockers, and calcium antagonists (Deanfield et al. 1984).
Effects on Blood and Arteries
1.
Platelets. Platelet hyperaggregation seen in patients with angina or induced by catecholamines can be normalized by propranolol. The second stage of platelet aggregation, induced by adenosine diphosphate, catecholamines, collagen, or thrombin, can be abolished or inhibited by propranolol. Propranolol is able to block [14C]serotonin released from platelets and inhibits platelet adherence to collagen; these favorable effects can be detected with the usual clinical doses of propranolol and other beta-blocking drugs.
2.
HDL-C. It has been suggested that beta-blockers may increase atherosclerosis by decreasing HDL levels. Propranolol causes a mild decrease in HDL levels of ~7 %. There is at present no proof that decreasing HDL values from, for example, 55 to 50 mg/dL (i.e., from 1.4 to 1.3 mmol/L) will have any adverse effect on the progression of atherosclerosis. Some studies suggest that HDL2 remains unaltered (Valimaki and Harno 1986; Pasotti et al. 1986). There is little doubt that in some patients HDL2 is slightly lowered. In one study, there was an 8 % lowering of HDL2 and a rise in triglycerides produced by both propranolol and pindolol at 6 weeks. Beta-blockers do not decrease total serum cholesterol. The effect of beta-blockers on triglycerides is variable, and the evidence associating raised triglycerides with ischemic heart disease (IHD) is weak. Acebutolol with weak ISA causes no significant disturbance of lipid levels. Bisoprolol, being highly beta1-selective, does not significantly decrease HDL-C.
3.
Arteries. Beta-blockers decrease the force and velocity of cardiac contraction, decrease RPP and heart rate × peak velocity, and therefore decrease hemodynamic stress on the arterial wall, especially at the branching of arteries. This action may decrease the atherosclerotic process and plaque rupture. This beneficial hemodynamic effect, and that described on blood coagulation, may favorably influence atherosclerotic CHD and subsequent occlusion by platelets or thrombosis.
4.
Coronary blood flow. Beta-blockers increase diastolic coronary perfusion time and coronary blood flow because bradycardia lengthens the diastolic filling time. Thus, these agents produce beneficial effects in angina and IHD by increasing coronary blood supply while reducing myocardial oxygen demands; the reduction of hydraulic stress in the coronary arteries appears to provide protection from plaque fissuring and rupture (see Fig. 1-1).
Effect on Serum Potassium
Beta-blockade causes a mild increase in serum potassium because of blockade of the beta2-mediated epinephrine activation of the Na+, K+—ATPase pump, which transports potassium from extracellular fluid into cells.
During stress, serum potassium has been observed to decrease up to 1.0 mEq (mmol)/L; this fall in serum potassium concentration can be prevented by blockade of beta2-receptors.
Non-cardioselective agents are superior to selective agents in preventing fluctuations of serum potassium concentration during stress and possibly during acute MI.
Among inpatients with AMI, the lowest mortality was observed in those with post-admission serum potassium levels between 3.5 and <4.5 mEq/L compared with those who had higher or lower potassium levels (Goyal et al. 2012).
Salutary Effects of Beta-Adrenergic Blockade
These beneficial effects include the following:
A decrease in heart rate increases the diastolic interval and allows for improved diastolic filling of the coronary arteries. This effect is especially important during exercise in patients with angina.
The RPP is decreased, so there is less myocardial demand for oxygen, resulting in an improvement of ischemia.
A decrease in sudden cardiac death has been documented in several studies. An impressive 67 % reduction in sudden death was observed in smokers and nonsmokers in the Timolol Norwegian Reinfarction Study (16): timolol decreased the overall mortality rate by 36 %, p < 0.001.
This beneficial effect of beta-blockers in post-infarction patients was reconfirmed in the Beta-Blocker Heart Attack Trial (BHAT). This well-run trial randomized 16,400 post-MI patients to propranolol or placebo and after 2-years follow-up showed a 26 % reduction in the mortality rate with propranolol.
A decrease in fatal arrhythmias and an increase in VF threshold, as well as amelioration of bothersome benign ventricular and supraventricular arrhythmias, have been established by several clinical studies.
A decrease in the velocity and force of myocardial contraction results in a decrease in myocardial oxygen requirement and also reduces the rate of rise of aortic pressure, which is important in the prevention and treatment of aortic dissection. Beta-blockade is effective in slowing the rate of aortic dilation and reducing the development of aortic complications in patients with Marfan’s syndrome (Shore et al. 1994)
A decrease in ejection velocity reduces hydraulic stress on the arterial wall that could be crucial at the site of atheroma. This mechanism of action may reduce the incidence of plaque rupture and may thus protect patients from coronary thrombosis and fatal or nonfatal infarction.
Beta-blockers may prevent early morning platelet aggregation induced by catecholamines and may decrease the early morning peak incidence of acute MI (Peters et al. 1990).
The favorable effect of beta-blockade on sudden death relative to reduction in heart rate is observed only with the use of beta-blockers that reduce heart rate (Singh 1990). Beta-blockers such as pindolol with marked ISA and no bradycardic response at rest cause no reduction in sudden death or mortality rates Acebutolol with mild ISA, however, caused a 48 % reduction in overall mortality rate and a 58 % reduction in cardiovascular mortality rate in post-infarction patients (Boissel et al. 1990).
Beta-Blockers Versus Calcium Antagonists and Oral Nitrates
The clinical effects of beta-blockers compared with calcium antagonists and oral nitrates are shown in Table 1-1.
1.
A decrease in heart rate by beta-blockers allows for a longer diastolic filling time and therefore greater coronary perfusion.
2.
It is often stated that beta-blockers may decrease coronary blood flow, but this is secondary to the reduction in myocardial work; in practice, this effect is not harmful. A decrease in blood flow does not occur if there is ischemia, and therefore it is not of importance in occlusive coronary disease. If you need less oxygen, you will need less blood flow; this fact is often misinterpreted. The RPP at rest and on maximal exercise is reduced by beta-blockers, but it is not decreased by calcium antagonists or oral nitrates.
3.
Both beta-blockers and calcium antagonists have been proved to be more effective than nitrates when they are used alone in the relief of angina pectoris.
4.




In animals, blood flow to the subendocardial ischemic myocardium distal to an organic obstruction is improved by beta-blockers, and it may be decreased by calcium antagonists (Weintraub et al. 1982). Beta-blockers divert blood from the epicardium to the ischemic subendocardium by activation of autoregulatory mechanisms. Calcium antagonists may have the opposite effect and can cause deterioration in patients with critical coronary artery stenosis (Warltier et al. 1983). Unfortunately, calcium antagonists, when used without a beta-blocker in patients with unstable angina, can increase chest pain and infarction, and they appeared to increase mortality in the largest subgroup of patients with unstable angina. Oral nitrates have an effect similar to that of calcium antagonists.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


