Beta-Blocker Therapy for Heart Failure
Henry Krum
Beta-adrenergic blocking agents (beta-blockers) are now well-established as mandatory therapy in patients with systolic chronic heart failure, unless not tolerated or contraindicated. Use of beta-blockers for the treatment of chronic heart failure has quite literally been a revolution in pharmacological therapy for this condition. These agents have long been contraindicated in the treatment of patients with systolic heart failure, the clinical consequence of impaired myocardial contractile function. However, in the late 1970s and early 1980s Swedish researchers tested the hypothesis that blockade of chronic sympathetic activation might, in fact, be beneficial in this setting. Decades later, we now have an extensive clinical trials database demonstrating the benefits of these agents in improving prognosis, reducing hospitalization, and (particularly in more severe cases) relieving symptoms. This database of placebo-controlled beta-blocker trials in congestive heart failure (CHF) is now more extensive than that for angiotensin-converting enzyme (ACE) inhibitors, which is standard background therapy for this condition. The robustness of this data is reflected by current heart failure guidelines that mandate the use of beta-blockers if tolerated in patients with mild, moderate, and severe symptoms of systolic heart failure, provided the patient has been stabilized and rendered euvolemic.
Pathophysiological Considerations
Role of the Sympathetic Nervous System in Heart Failure
Chronic heart failure is characterized by activation of a number of neurohormonal vasoconstrictor systems including the sympathetic nervous system, the renin-angiotensin system, vasopressin, and, more recently, peptide systems such as endothelin and urotensin-II (1,2). This neurohormonal activation can be viewed as a compensatory response to the reduction in cardiac output and systemic blood pressure that accompanies systolic ventricular dysfunction.
Beta-adrenoceptor agonism activates regulatory G-proteins to increase intracellular cyclic adenosine monophosphate (AMP) via adenylate cyclase stimulation (3). Increased cyclic AMP (cAMP) stimulates downstream protein kinases which, in turn, phosphorylate calcium channels leading to influx of intracellular calcium, and enhancement of coupling of actin and myosin filaments, resulting in inotropy (Fig. 28-1).
While there is generalized sympathetic activation in heart failure, norepinephrine spillover is particularly increased in the heart and kidneys (4). As a consequence of the chronic catecholamine excess that accompanies this activation, there is depletion of catecholamines from storage vesicles in cardiac sympathetic nerve terminals (5), downregulation (decreased density) of beta-adrenoceptors on myocardial cells, and uncoupling of the receptor from adenylate cyclase (3).
In the short term, the sympathetic activation that accompanies heart failure is an important compensatory mechanism providing inotropic support and maintaining cardiac output; indeed, that is the rationale for the use of sympathomimetic amines in patients with acutely decompensated heart failure. However, over longer periods of time this sympathetic activation may be deleterious, thus providing the rationale for use of beta-adrenoceptor-locking agents in this setting. There are several lines of evidence to support the conclusion that long-term sympathetic activation is detrimental in heart failure and
contributes to disease progression and adverse clinical outcomes.
contributes to disease progression and adverse clinical outcomes.
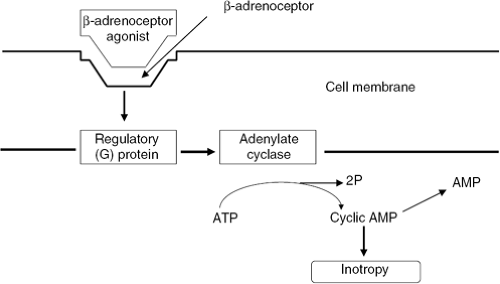 Figure 28-1 The β-adrenegic receptor system. ATP, adenosine triphosphate; AMP, adenosine monophosphate. |
Evidence from Mechanistic Studies
Possible detrimental effects of long-term sympathetic activation derived from mechanistic studies in chronic heart failure include direct myocardial toxicity secondary to long-term catecholamine excess (6), requirement for increased myocardial oxygen consumption (7), myocardial beta-adrenoceptor3 downregulation, impaired myocardial function secondary to tachycardia, reduction in threshold for arrhythmogenesis (8), and activation of other vasoconstrictor systems such as the renin-angiotensin improvement in endothelial function and endothelin systems.
Evidence from Clinical Outcome Studies
There are also a number of lines of evidence supporting the deleterious effects of chronic sympathetic activation derived from clinical studies. It has long been observed that patients with diseases associated with chronic catecholamine excess (e.g., pheochromocytoma) present with myocardial disease phenotypically indistinguishable from that of dilated cardiomyopathy (9).
Drugs that augment the effects of catecholamines on the myocardium such as beta-adrenoceptor agonists (10) or type III phosphodiesterase inhibitors (11) are associated with adverse mortality outcomes. This was perhaps best demonstrated in the PROMISE study (11) with long-term oral use of the phosphodiesterase inhibitor, milrinone, associated with significantly impaired survival. Similar outcomes were associated with the partial beta-adrenoceptor agonist, xamoterol (10). In contrast to these dismal results with long-term oral treatment, milrinone remains a useful short-term intravenous therapy, providing inotropic support to the failing myocardium in decompensated heart failure (12). A number of studies have demonstrated a close association between measures of sympathetic activation (peripheral venous plasma norepinephrine levels, whole-body norepinephrine spillover rate, and microneurography) with markers of disease severity and subsequent mortality (13,14).
Finally, the accumulating evidence from studies of beta-adrenoceptor-blockers themselves in heart failure supports the underlying rationale for their use: that blockade of the toxic effects of catecholamines on the myocardium is associated with improved clinical status and survival in these patients.
Rationale for Adrenergic Blockade in Patients with Chronic Heart Failure
The preceding mechanistic and clinical data regarding long-term sympathetic activation in chronic heart failure strongly support a therapeutic role for beta-adrenoceptor-blockers in this condition. Theoretical benefits of beta-adrenoceptor-blockers include direct myocardial protection from the toxic effects of catecholamines, reduction in stimulation of other neurohormonal vasoconstrictor systems, blockade of the proarrhythmic effects of catecholamines and anti-ischemic effects via increased coronary blood flow during diastole, and reduced myocardial oxygen demand.
It has also been proposed that restoration of beta-adrenoceptor density and receptor-adenylate cyclase coupling with the use of these drugs may be a potential mechanism for their beneficial effects. Myocardial beta-adrenoceptor downregulation and receptor-cyclase uncoupling in heart failure may limit contractile function (3,15), and drugs such as metoprolol have been demonstrated to increase beta-adrenoceptor density in this setting (16). However, other beta-adrenoceptor-blocking drugs have been demonstrated to produce significant clinical benefits in this condition (e.g., carvedilol) and are not associated with beta-adrenoceptor upregulation or restoration of receptor-cyclase coupling (17). Thus, although some beta-adrenoceptor-blockers do cause upregulation of beta-adrenoceptors, this is unlikely to be the mechanism of benefit of this group of drugs overall.
Pharmacology of Beta-Blockers
Bisoprolol
Bisoprolol is a beta-1 selective agent without additional vasodilatory properties. Its beta-1 to beta-2 ratio makes it a particularly selective agent and thus, in theory, less likely to
contribute to bronchoconstriction in individuals with airways disease. The pharmacokinetics of the agent is that of a lipid-insoluble agent with a plasma half-life of 10 to 12 hours, permitting once-daily dosing. The target dose is generally 10 mg per day. Elimination is via both renal and hepatic routes.
contribute to bronchoconstriction in individuals with airways disease. The pharmacokinetics of the agent is that of a lipid-insoluble agent with a plasma half-life of 10 to 12 hours, permitting once-daily dosing. The target dose is generally 10 mg per day. Elimination is via both renal and hepatic routes.
Carvedilol
Carvedilol is a moderately lipid-soluble, nonselective (beta-1, beta-2) receptor antagonist without intrinsic sympathomimetic activity (ISA) but with the additional property of alpha-1 blockade, mediating peripheral vasodilation. It also possesses ancillary properties of being antioxidant, antiproliferative, and having antiendothelin actions. Its half-life is 6 to 10 hours and twice-daily dosing is recommended, although a prolonged-release formulation is currently being developed. Elimination is primarily hepatic and dose adjustment is not required in renal impairment. The target dose is 25 mg twice daily (50 mg twice daily in patients >85 kg).
Metoprolol
Metoprolol is a mildly lipid-soluble beta-1 selective agent without ancillary properties or vasodilator activity. It comes in an immediate-release formulation with a half-life of 3 to 5 hours, requiring two- to three-times-daily dosing, as well as an extended-release formulation permitting once-daily dosing. The target dose in heart failure of the extended-release formulation is 200 mg per day.
Nebivolol
Nebivolol is a lipophilic beta-1 selective agent that has additional vasodilator properties mediated via nitric oxide donation. Based on the Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors (SENIORS) study (18), its target dose is 10 mg per day.
Other Beta-Blocking Agents
A number of other beta-blockers have been studied in heart failure, including bucindolol. Bucindolol is a nonselective beta-1 receptor antagonist. There is ongoing debate as to whether bucindolol also possesses ISA (19,20); this has been observed in both animal studies and in failing human myocardium. The significance of the presence of ISA is that of adverse outcomes with agents that possess this property, including the beta-1 partial agonist xamoterol (10), as well as non—beta-blocking agents that act via cAMP, the second messenger of the beta-adrenoceptor vesnarinone (21), flosequinan (22), ibopamine (23).
Efficacy of Beta-Blockers in Heart Failure
Studies of Clinical Status/Surrogate Markers
The pioneering clinical observations of Swedish and other investigators (24,25,26) established the utility of beta-blockers in heart failure. However, many of these early studies were often uncontrolled and involved administration of drugs for only short periods of time. Nevertheless, the clinical experience and data obtained from these trials have subsequently proven invaluable, leading to larger, placebo-controlled studies and the widespread clinical use of these drugs.
Short-term use of beta-adrenoceptor-blockers in these early studies was found to result in neutral or adverse clinical outcomes (27,28) and led many investigators to abandon this therapeutic approach. However, it is now understood that in the context of recovery of the myocardium from chronic catecholamine stimulation, studies of at least 3 months’ duration are generally required to demonstrate clinical benefit with these drugs. Early studies were also important in establishing the need for commencement of beta-adrenoceptor-blocker therapy at extremely low doses to avoid sudden interference with the inotropic support provided to the failing myocardium by the increased sympathetic activity that accompanies heart failure.
Since these early trials, a number of longer-term, double-blind, placebo-controlled studies of beta-adrenoceptor-blockers have assessed clinical status in patients with chronic heart failure (28,29,30,31,32). These studies have demonstrated consistent improvements in ejection fraction, usually accompanied by improved patient well-being but with variable effects on exercise tolerance. The lack of consistent benefit of beta-adrenoceptor-blockers on exercise tolerance is a feature of therapy with this drug class and relates to the heart rate-limiting effects of beta-adrenoceptor-blockade during exercise. Heart rate is one of the factors that determines maximal exercise capacity (VO2max) and, as such, it is not surprising that beta-adrenoceptor-blockers do not lead to increases in VO2max, despite the hemodynamic improvements that accompany use of these drugs. These studies also demonstrated that beta-adrenoceptor-blockers inhibit other neurohormonal systems activated in chronic heart failure, such as the renin-angiotensin system (29) and endothelin-1 (33), as well as restoring parasympathetic activity (34), which is impaired in this condition.
The beneficial clinical effects observed with beta-adrenoceptor-blockers in heart failure in single-center studies has led to the more widespread evaluation of these drugs. The Carvedilol U.S. study program (35) addressed multiple questions regarding clinical efficacy of this drug in heart failure: use in delaying progression of heart failure, clinical utility in patients with moderate to severe heart failure, and whether a clinical dose-response relationship existed. The overall findings were concordant with those of the previous single-center studies with the drug. Carvedilol significantly improved left ventricular ejection fraction, New York Heart Association (NYHA) functional class, and physician and patient global assessment of heart failure status. Improvements in left ventricular function were found to be dose-dependent, the greatest benefits being seen in those patients randomized to receive the highest dose of the drug.
Retardation of progression of disease with carvedilol was also noted in the Australian New Zealand Heart Failure Study (36), where a reduction in left ventricular chamber size was observed in mild heart failure patients treated with beta-adrenoceptor-blocker, indicating restoration of ventricular contour toward normal. This finding
provided strong a priori evidence that excess catecholamines are important in the ventricular remodeling process in heart failure and that beta-adrenoceptor-blockers can interfere with this remodelling in a clinically significant manner. However, significant improvements in NYHA class were not seen in this very mild group of patients, 30% of whom were already NYHA class I (i.e., asymptomatic) at time of entry into the study.
provided strong a priori evidence that excess catecholamines are important in the ventricular remodeling process in heart failure and that beta-adrenoceptor-blockers can interfere with this remodelling in a clinically significant manner. However, significant improvements in NYHA class were not seen in this very mild group of patients, 30% of whom were already NYHA class I (i.e., asymptomatic) at time of entry into the study.
Studies on Mortality and Hospitalization
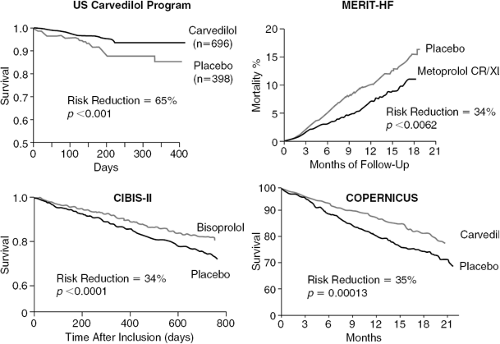
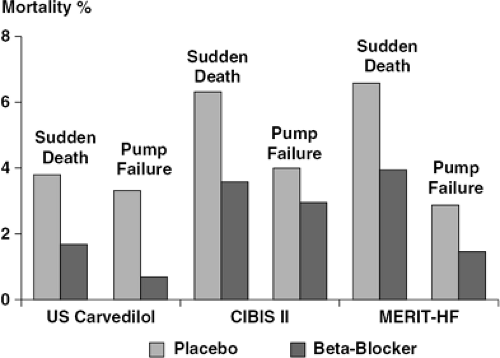
Chronic sympathetic activation is associated with adverse mortality outcomes in heart failure. Patients with the highest plasma levels of norepinephrine (a crude marker of sympathetic activation) have the most adverse mortality outcomes. Because sympathetic activation is also a marker of disease severity, the mortality association may simply reflect patients with the most advanced disease having the greatest mortality. As previously described, chronic sympathetic stimulation is associated with proarrhythmia, direct trophic and toxic effects on the myocardium, and activation of other neurohormonal systems, all of which may independently lead to adverse mortality outcomes. Thus, it would be reasonably anticipated that use of these drugs should confer beneficial effects on mortality, providing a powerful rationale for prescribing such drugs in these patients. A number of placebo-controlled studies have now conclusively demonstrated beneficial effects on mortality and hospitalization (Figs. 28-2 and 28-3).
Studies of Carvedilol
U.S. Carvedilol
This was a study of carvedilol versus placebo on a number of surrogate endpoint studies that spanned systolic CHF severity from mild through to severe (35). The pooled mortality result (prespecified) demonstrated a 65% risk reduction, resulting in the Data Safety and Monitoring Board (DSMB) terminating the study early. The magnitude of this benefit appeared to be similar in patients with mild through severe symptoms, regardless of idiopathic, dilated, or ischemic cardiomyopathy etiology. There were only a small number of deaths recorded in the U.S. Carvedilol study, leading some observers to conclude that a definitive mortality effect with beta-adrenoceptor-blockers had not been adequately demonstrated. There were also highly significant effects on hospitalization.
ANZ Carvedilol
Similar observations were made in the ANZ Carvedilol study (36). This was a study of mild heart failure patients, all with ischemic etiology. Although this study was not powered to detect a morbidity/mortality outcome, the findings were a 41% reduction in death or all-cause hospitalization and a 25% reduction in mortality alone.
COPERNICUS Study
This was a placebo-controlled study of carvedilol in patients with advanced heart failure (37). Although NYHA Class was not assessed in COPERNICUS, patients within this subgroup were selected on the basis of advanced heart failure symptoms together with an ejection fraction of >25%. Patients were permitted to have been recently hospitalized and to have received intravenous inotropic therapy within 4 days of randomization. The 2,289 patients were included in the intent-to-treat analysis, with a 35% decrease in mortality at the time the study was stopped early by its DSMB.
Studies of Bisoprolol
There have been two studies exploring the effects of bisoprolol on mortality in comparison to placebo. These were focused on patients with moderate to severe heart failure.
The first Cardiac Insufficiency Bisoprolol Study (IBIS) (38) study of 641 patients noted a 20% reduction in the primary endpoint of mortality and a 34% reduction in hospitalizations for heart failure. However, the primary endpoint did not reach statistical significance and clearly, in retrospect, this was an underpowered study. This led to the larger CIBIS II study (39) of 2,647 patients. This study was also terminated early by its DSMB for a 34% risk reduction with an accompanying 36% reduction in hospitalization for worsening heart failure.
Studies of Metoprolol
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree