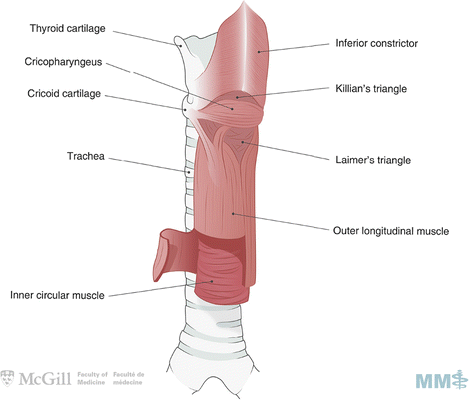
Fig. 8.1.
Esophageal anatomy. Used with permission from the McGill University Health Centre Patient Education Office.
Table 8.1.
Vasculature, lymphatics, and innervation of the esophagus.
Cervical esophagus | Thoracic esophagus | Abdominal esophagus | |
|---|---|---|---|
Arterial supply (segmental) | • Inferior thyroid artery • Superior thyroid artery (cricopharyngeus muscle) | • Aorta (4–6 esophageal branches) • Left/right bronchial arteries (esophageal branches) • Branches of: inferior thyroid, intercostal, inferior phrenic arteries | • Left gastric artery • Inferior phrenic arteries |
• All arterial branches terminate in a capillary network forming rich collaterals outside and within the submucosa, along the entire length of the esophagus → Allows the esophagus to be surgical mobilized with a low risk of ischemia | |||
Venous supply (segmental) | • Inferior thyroid veins | • Venae comitantes (surround esophagus) → Azygos, hemiazygos veins | • Inferior phrenic veins • Left gastric vein (coronary vein), short gastric veins |
• First basin of venous drainage is the rich submucosal venous plexus which drains into the superficial periesophageal venous plexus → Allows for very rapid spread of infection and tumor to three body cavities | |||
Lymphatic drainage (non-segmental) | • Paratracheal nodes • Deep cervical, internal jugular nodes | • Superior and posterior mediastinal nodes (paratracheal, subcarinal, paraesophageal, retrocardiac, infracardiac, para-aortic, inferior pulmonary ligament nodes) | • Gastric and celiac nodes |
• Extensive lymphatic networks in the lamina propria, submucosa, muscularis propria, and adventitia → Allows for very rapid spread of infection and tumor to three body cavities • Very extensive longitudinal and transmural drainage—unpredictable lymphatic spread | |||
Innervation | • Vagus nerve: Recurrent and superior laryngeal nerve • Cervical sympathetic trunk | • Vagus nerve: Direct branches • Thoracic sympathetic trunk | • Vagus nerve: Anterior/posterior plexus • Greater and lesser splanchnic nerves |
• Vagus nerve provides motor, sensory, parasympathetic, and sympathetic innervation • Injury to the RLN can lead to dysmotility, hoarseness, and aspiration • Intrinsic innervation: Myenteric (Auerbach) plexus and submucosal (Meissner) plexus | |||
Physiology
Swallowing: Oropharyngeal Phase (1.5 s)
1.
Elevation of tongue—bolus pushed into posterior oropharynx (voluntary)
2.
Posterior movement of tongue—bolus pushed into hypopharynx (voluntary)
3.
Elevation of soft palate—close off passage into nasopharynx
4.
Elevation of hyoid—brings epiglottis under tongue
5.
Elevation of larynx—opens retrolaryngeal space
6.
Tilting of epiglottis—covers opening of larynx
Swallowing: Esophageal Phase
Upper Esophageal Sphincter
High-pressure region between pharynx and esophagus, tonically contracted at 60 mmHg (2–3 cm length) via the cricopharyngeus muscle to prevent reflux and aspiration
During swallowing, upper esophageal sphincter (UES) relaxes, while posterior pharyngeal constrictors propel food boluses into the esophagus using a pressure differential between the cervical esophagus (positive pressure) and intrathoracic esophagus pressures (negative pressure)
UES subsequently closes, reaching 90 mmHg pressure for 2–5 milliseconds, marking the start of peristalsis
UES relaxes back to its resting tonic pressure of 60 mmHg
Peristalsis
• Primary
Progressive contractions, moving down the esophagus at 2–4 cm/s
Reaches LES 9 s after initiation of voluntary swallowing
Intraluminal pressure: 40–80 mmHg
• Secondary
Progressive contractions initiated by distension or irritation of esophagus (not voluntary swallowing)
Clears esophagus of leftover food from primary peristalsis
• Tertiary
Uncoordinated, nonprogressive contractions
Responsible for esophageal spasm
Lower Esophageal Sphincter
Not a true sphincter; corresponds only to a slight thickening of the esophageal muscularis layer
High-pressure zone 2–5 cm in length, resting tonic pressure 6–26 mmHg (minimum 1 cm intra-abdominal for normal lower esophageal sphincter (LES) function) as barrier to reflux
Respiratory inversion point (RIP): Transition from intrathoracic to intra-abdominal LES
Transient relaxation of 5 s, occurring 2–3 s after oropharyngeal phase
Gastroesophageal Reflux Disease
Pathophysiology
Abnormal flow of gastric content into the esophagus, secondary to abnormalities of:
LES
Esophageal peristalsis
Hiatal hernia affecting competency of GEJ
Mucosal injury leads to pain, cough, heartburn, dysphagia, and regurgitation
Management: Nonsurgical
Lifestyle interventions: weight loss, elevation of the head of the bed, avoidance of meals before bedtime, avoidance of foods that decrease LES tone
Medical:
Antacids (calcium carbonate, sodium bicarbonate, aluminum and magnesium hydroxide):
Provide rapid short-term relief, but limited role for the treatment of erosive esophagitis
Histamine 2 receptor antagonists:
Can heal mild esophagitis, or adjunct to proton-pump inhibitors (PPI) for acid breakthrough
PPI (mainstay of treatment):
Superior healing rates and decreased relapse rates for erosive esophagitis and non-erosive reflux disease compared to other medical therapy
Prokinetic agents (domperidone, metoclopramide):
Useful in patients with delayed emptying and gastroparesis
Sucralfate:
Binds to mucosa, preventing injury
GABA-B agonist (baclofen):
Use is very limited due to side-effect profile on the central nervous system
Management: Antireflux Surgery
Antireflux surgery (ARS) is more effective than medical management for treating gastroesophageal reflux disease (GERD) [1].
However, given the efficacy of medical management and its limited side-effect profile, first-line treatment for GERD is PPI with lifestyle modifications.
Indications of ARS
Failed medical management
Patients who cannot take long-term PPIs (e.g., cost, compliance, side effects)
Patients with respiratory symptoms or vocal cord damage secondary to reflux
Although many patients have regression of metaplasia (Barrett’s esophagus), it is generally not performed as a cancer-prevention strategy [2].
Objective evidence of GERD is required prior to proceeding with ARS—e.g., 24-h pH monitoring or reflux esophagitis on endoscopy.
Goal of ARS is to restore a competent LES.
Options: Laparoscopic fundoplication
Nissen: 360° wrap posteriorly (complete wrap)
Toupet: 270° posteriorly (partial wrap)
Dor: 180° anteriorly (partial wrap)
Hill: 180° posteriorly (partial wrap)—rarely performed due to anchoring to preaortic fascia and the risk of vascular injury
Partial wraps have decreased postoperative dysphagia compared to Nissen fundoplication and thought to be a better option for patients with motility disorders (e.g., achalasia)—however this is controversial.
Technical elements:
Division of short gastric vessels
Extensive posterior mediastinal dissection (mobilize GEJ >2 cm intra-abdominally)
Closure of crura using a bougie
Securing fundoplication (gastropexy)
Treatment of GERD in the context of morbid obesity is best managed with bariatric procedures.
Paraesophageal Hernia
Hiatal Hernia (HH) Classification (Fig. 8.3)
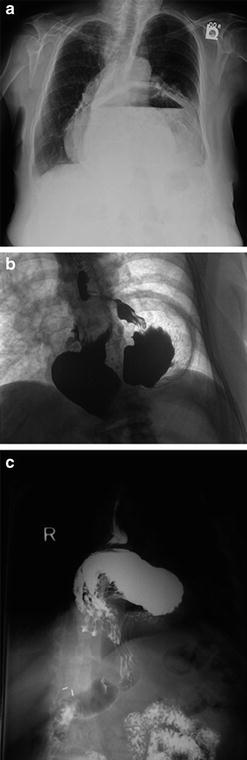
Fig. 8.3.
Chest X-ray (a) and upper GI contrast studies (b, c) of Type III paraesophageal hernias.
1.
Type I (Sliding HH): most common (>95 %); intra-abdominal GEJ → intrathoracic
2.
Type II paraesophageal hernia (PEH): Least common; GEJ at its normal anatomical location, but an enlarged esophageal hiatus allowing the stomach fundus to herniate into the thoracic cavity
3.
Type III PEH: Combination of Type I and II, with displaced GEJ and stomach herniation through the hiatus
4.
Type IV PEH: Herniation of other intra-abdominal organs into the thoracic cavity (spleen, colon, omentum, etc.)
Pathophysiology
Increased age: Loss of elasticity and weakening of the phreno-esophageal ligament
Increased intra-abdominal pressure (due to obesity, ascites, chronic cough, pregnancy)
Gastric Volvulus
Stomach most common organ to herniate and is occasionally associated with gastric volvulus
Organoaxial volvulus
Stomach rotated around the axis from the phrenoesophageal membrane to the pylorus
Antrum rotates creating an “upside-down stomach”
60 % of all gastric volvulus
Associated with PEH and high rates of strangulation and necrosis (5–28 %)
Mesoenteroaxial volvulus
Stomach rotation around axis which bisects greater and lesser curve
Posterior stomach becomes anterior
Not always associated with PEH
Clinical Presentation
Intermittent solid food dysphagia (acute obstruction)
Intermittent and postprandial epigastric or chest pain (50 %)
Acute severe pain: Need to rule out gastric volvulus and strangulation
GI bleeding from gastric ischemia → mucosa ulceration (Cameron’s ulcer); chronic iron-deficiency anemia
Strangulation
Severe and persistent pain, +/− fever, retching without vomiting
Evaluation
Esophagogram:
Diagnosis and anatomical delineation of stomach relative to diaphragm
Rule out volvulus or strangulation
Esophagogastroscopy:
Direct evaluation of gastric mucosa during strangulation
Rule out other pathology
Manometry and 24-h pH studies are of limited use in large hiatal hernias
CT scan (particularly coronal images)
Management (Fig. 8.4)
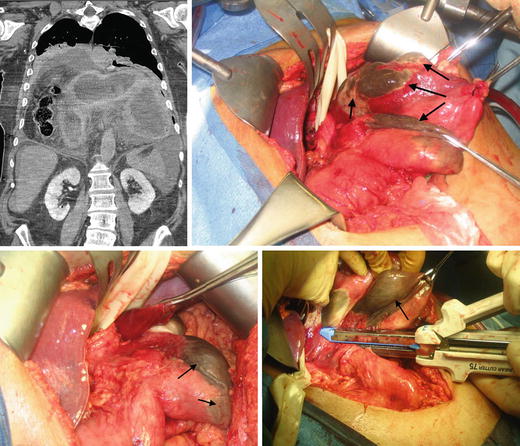
Fig. 8.4.
Strangulated PEH with a nonviable stomach fundus (black arrows showing areas of necrosis). This patient underwent a sleeve gastrectomy.
Approach: Laparoscopic/transabdominal, thoracoscopic/transthoracic
Similar recurrence rates; shorter length of stay, lower morbidity and pain using laparoscopy
Laparoscopic PEH repair is currently the standard of care for all primary hiatal hernias. Open transthoracic approaches may have a role in recurrent cases.
Principles of PEH repair
1.
Hernia reduction
2.
Hernia sac dissection +/− resection
3.
Hernia defect closure
4.
Antireflux procedure to prevent high rates of postoperative reflux
5.
GEJ restoration to normal anatomic location
Strangulation
Immediately decompress stomach with nasogastric tube
Surgical emergency: Necrosis, perforation → mediastinitis; 50–80 % mortality
Viable stomach: Repair PEH with fundoplication
Although feasible via laparoscopic approach, the strangulated PEH may require open repair to reduce the stomach delicately if the gastric wall is edematous/inflamed.
Nonviable stomach (Fig. 8.5): Partial vs. total gastrectomy, depending on the degree of necrosis
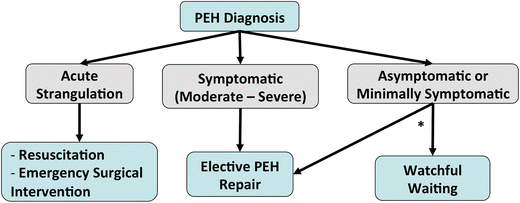
Fig. 8.5.
Management algorithm for paraesophageal hernias (PEH). *Patient preference and performance status are considered when deciding if asymptomatic or minimally symptomatic patients should undergo elective repair.
Typically, the fundus is the only necrotic component, thereby sparing the lesser curvature and allowing for a sleeve gastrectomy
Surgery in asymptomatic or minimally symptomatic patients
Controversial—watchful waiting vs. elective repair of all PEH irrespective of symptoms
Annual risk of acute strangulation: 1.1 %; mortality of elective laparoscopic PEH repair: 1.4 % [7]
Although this reported rate of post-PEH mortality is significantly higher than most recent surgical series (<1 %)
Patient preference and performance status are considered when deciding if these patients should undergo elective repair. Currently elective repair is recommended in most patients of good performance status.
Complications
Recurrence: Despite high anatomic recurrence on follow-up imaging after laparoscopic PEH repair (up to 50 %), the majority of patients have minor/small recurrences that remain minimally symptomatic or asymptomatic [8].
Patients complaining of recurrent dysphagia, heartburn, or regurgitation should be reevaluated with an upper GI study and esophagogastroscopy to assess for recurrence, excessively tight fundoplication, or slipped wrap.
Recurrences causing significant quality-of-life impairment and slipped wraps should be treated operatively.
Dysphagia is not an infrequent early consequence after the repair of large PEH due to tissue edema. Although the majority of cases resolve, endoscopic balloon dilatation can be considered after 6 weeks for persistent symptoms.
Gastroparesis: Patients present with postoperative bloating. Evaluation is performed using gastric emptying study and esophagogastroscopy to rule out cancer and other pathologies—trial of pro-motility agents for persistent major bloating symptoms.
Use of Prosthetic Mesh (Biologic or Synthetic)
Synthetic mesh: Associated with complications, such as esophageal erosion, stricture, and ulceration, requiring re-operation for mesh removal and a non-insignificant risk of esophageal resection.
Biologic mesh: Not associated with the complications related to the use of synthetic mesh.
Several studies have looked at outcomes of mesh reinforcement compared to primary repair of PEHs (Table 8.2).
Table 8.2.
Outcomes of mesh reinforcement compared to primary repair of paraesophageal hernia repair.
Study
Methodology
Median follow-up (months)
Recurrence
Mesh-related complications
Synthetic mesh
Frantzides et al. (2002) [23]
RCT: PR (N = 36) vs. PTFE (36)
31
PR = 22 %; PTFE = 0 % p < 0.006
0 %
Zaninotto et al. (2007) [24]
Retrospective chart review PR (N = 19) vs. Goretex (35)
71
PR = 42 %; PTFE = 9 % p = 0.01
3 %
Biologic mesh
Ringley et al. (2006) [25]
Prospective non-randomized PR (N = 22) vs. AlloDerm (N = 22)
10
PR = 9 %; PTFE = 0 % (no statistical analysis)
0 %
Jacobs et al. (2007) [26]
Retrospective chart review PR (N = 93) vs. Goretex (127)
PR = 46 Surgisis = 38
PR = 20 %; Surgisis = 3 %
p < 0.01
0 %
Oelschlager et al. (2011) [9]
RCT: PR (N = 57) vs. Surgisis (51)
58
PR = 59 %; Surgisis = 54 % p = 0.7
0 %