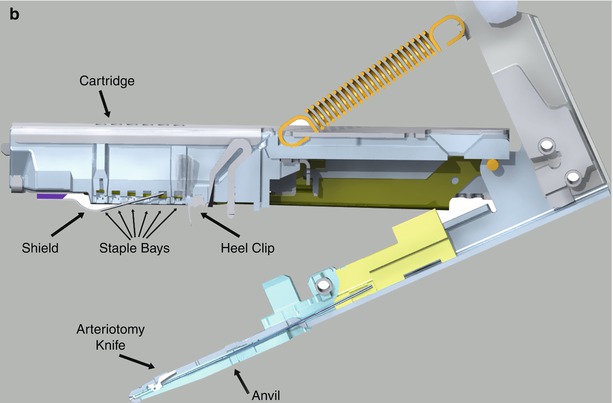
Fig. 9.1
Cardica Flex A™ graft to coronary artery anastomotic device. (a) The anastomotic end is equipped with a graft-stabilizing platform, an anvil that fits in the coronary arteriotomy, and a stapling mechanism. The cable driven firing mechanism is passed through a thoracic port to the anastomotic site. (b) Details of the anastomotic stapling end of the Flex A™ device (Courtesy Cardica, Inc.)
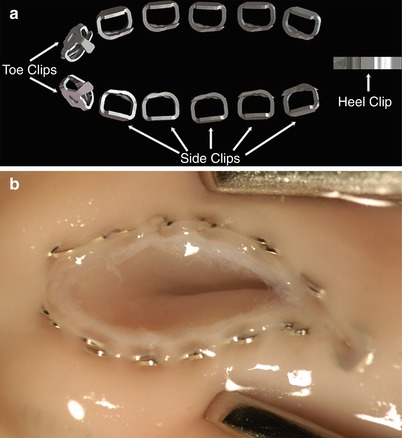
Fig. 9.2
(a) Flex A™ anastomotic staples. (b) Graft to Coronary Artery Staple Line—The stainless steel clips create a pliable interrupted anastomosis. The anvil is withdrawn from the completed anastomosis via a tiny hole at the heel, which is apposed with the pre-placed adventitial PTFE suture (Courtesy Cardica, Inc.)
Together these technology developments could become the “springboards” needed for wider adoption of true endoscopic robot-assisted revascularization. Moreover, both devices should extend the ability to perform multi-vessel coronary revascularization on a beating heart. These types of automated anastomotic devices also have been effective in performing proximal graft to aorta anastomoses in open chest cardiac surgery as well as in other vascular related specialties [3].
Patient Selection
Suitable patients for da Vinci™ endoscopic coronary artery grafting bypass should have good left anterior descending, diagonal branch, and/or high circumflex marginal coronary artery target vessels. The presence of diffuse coronary disease or arterial calcifications can present major impediments in using this type of mechanized anastomosis. Right coronary artery anastomotic sites are more difficult to access, unless there is an isolated proximal right coronary artery lesion in a region where right-sided instrument arm ports can be placed effectively. Patients who require emergent surgery, have poor cardiac function, and/or have had previous coronary surgery should not undergo a robot-assisted TECAB. The most common exclusionary criteria for performing TECAB surgery have been extensive left chest pleural adhesions and the patient’s inability to tolerate single lung ventilation as in patients with severe COPD.
Preparation and Anesthetic Considerations
The patient should be positioned supine on the operating room table with an elevating roll under the mid-left chest. The left arm should be tucked loosely, using a folded sheet, and be suspended slightly below the edge of the operating table. For intra-operative hemodynamic monitoring right radial arterial and pulmonary artery catheters are placed. Both legs should be prepared and draped, as in standard coronary bypass surgery, but with liberal groin exposure to enable rapid femoral cannulation for cardiopulmonary perfusion, should this become necessary. External defibrillator patches should be placed on both the left scapular area and the right lateral chest, subtending the maximal cardiac mass. Ventilation and single lung isolation are done using either a double lumen endotracheal tube or a single lumen tube with a left endo-bronchial balloon blocker.
Robot Instrument Arm Port Placement
Under endoscopic visualization, the camera port and robot instrument arms are then placed in the 2nd, 4th, and 6th intercostal spaces, centered between the mid-axillary and mid-clavicular lines (Fig. 9.3). Access to circumflex coronary artery marginal branches requires port placement slightly more posterior than described above. To maintain an adequate intra-thoracic workspace, continuous carbon dioxide insufflation is required to compress the lung after deflation. Two additional ports are required to perform the coronary anastomosis but should be placed after ITA mobilization and graft preparation are complete. For insertion of the 4th robotic instrument arm and the Endo-wrist™ stabilizer, a 12-mm port should be placed just medial to the mid-clavicular line in the left subcostal plane. For delivery of the Cardica Flex A™ anastomotic device, a 15-mm port (Ethicon Surgical, Somerville NJ) should be inserted in the 2nd interspace along the left mid-clavicular line. Thereafter, the robotic instrument cart should be positioned along the left side of the operating table and centered by the right instrument arm port. To allow better maneuvering of the 4th instrument arm, the center of the instrument cart may need to be oriented slightly more cephalad and angled toward the patient’s feet.
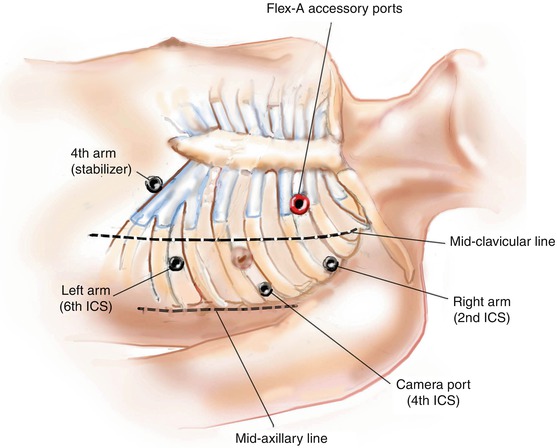

Fig. 9.3
Port placement. This illustration shows the best port (12-mm) placement sites for insertion of the camera (4th intercostal space, ICS), robotic instrument arms (2d and 6d ICS) and the Endostabilizer ™ (left subcostal, medial to mid-clavicular line). The 15 mm port for introduction of the Flex. A™ device is placed in the 2nd ICS medial to mid-clavicular line for all graft locations
Surgical Technique
Coronary Artery and Conduit Preparation
Using robotic forceps and a spatula electro-cautery, the pericardial fat pad should be dissected free and reflected laterally. For LAD grafting the pericardium should be opened anterior to the phrenic nerve, and for circumflex marginal coronary targets the pericardiotomy should be entered posterior to the phrenic nerve. After the pericardium is opened, careful angiographic review is helpful to identify the correct coronary target vessels. Once the planned vessel is identified and found to be suitable for endoscopic grafting, ITA graft dissection should be completed and the graft divided carefully, maintaining the correct pedicle orientation. If the right ITA is to be used, it should be mobilized first. A 0° robotic endoscope provides the best view, when dissecting substernal mediastinal fibro-fatty tissue and entering the right pleural space. The 30° endoscope should be used to harvest the right ITA while using low radiofrequency settings for the robotic electrocautery spatula. “Skeletonizing” (i.e., removing the associated fascia and intercostal musculature) the ITA is preferred to increase conduit length and help to maneuver the graft inside the thoracic cavity. Nevertheless, many surgeons still prefer to prepare the ITA as pedicle graft. Robotic micro-bipolar cautery forceps, set at low energy levels, are helpful for cauterizing small branches. Larger branches should be divided between robotically applied metal clips.
After ITA grafts are harvested, the port should be placed for the 4th robotic arm and introduction of the EndoWrist ™ stabilizer. Of note, docking the 4th arm (subcostal port) may require “setup joint” adjustment to cephalad of both the left robotic and camera arm. Prior to heparinization, the coronary artery target site should be stabilized. Coronary artery graft site preparation entails opening the overlying epicardium using low cautery energy and isolating the vessel proximally with a silastic snare. At this time an advential stitch (PTFE with CV8 needle) should be preplaced to later close the planned insertion site of the Flex A™ anvil after the anastomosis has been completed. To allow the EndoWrist ™ stabilizer to clear the cardiac apex, when being applied to posterior coronary arteries, it is important to position the 4th arm port slightly more laterally than if working on the anterior surface of the heart.
The patient should be heparinized at this time with a target activated clotting time of 300–350 s. A small bulldog clamp should be placed on the proximal ITA with the distal end occluded using a metal clip. Just proximal to this clip, the ITA then is transected partially with robotic fine scissors. Through a subcostal port, the blunt tip of an epidural catheter should be inserted carefully into the ITA lumen to achieve maximal graft dilation with a papaverine injection. To confirm proper intra-luminal catheter position, the tableside assistant should withdraw arterial blood prior to infusing the papaverine. Although less effective, papaverine may be injected along the ITA surface.
The planned grafting strategy dictates a defined set of operative next steps and grafting sequence. We recommend grafting the posterior coronary arteries first, if the patient can tolerate this maneuver from a hemodynamic standpoint. When a right internal thoracic artery (RITA) is being used as a “free” T graft, it should be detached at this point. To occlude the divided RITA proximally, either a large pedicle clip or several smaller metal clips can be deployed through a port prior to inserting the Cardica Flex A™ device. The T graft anastomosis should be performed “side to side” by attaching the proximal end of the free RITA to the LITA. We load the proximal end of the free RITA onto the device inside the chest and insert the anvil at the site of an ITA branch and control any site bleeding with two or three Nitinol U clips (Medtronic, Minneapolis, MN). Using this configuration, the free RITA is used to graft circumflex branches with the LITA applied to the LAD. If the LAD stenosis is fairly proximal, the RITA can remain attached “insitu” to the right subclavian artery and brought across the midline to bypass graft the LAD. To avoid re-operative injury of the trans-sternal (midline) RITA, some of the endothoracic fascia should be used to cover the graft.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


