Relative risk
95 % Confidence interval
P value
Overall
1.48
1.25–1.74
<0.001
CAD
1.46
1.31–1.63
<0.001
Stroke
1.35
1.19–1.54
<0.001
All-cause mortality
1.19
1.05–1.34
0.005
The FRS is a very useful tool to estimate the 10-year cardiac event risk in patients and is supported by the 2010 ACCF/AHA guideline [6] for assessment of cardiovascular risk in asymptomatic adults (ACCF/AHA class I, LOE B) by incorporating age, sex, total cholesterol, high-density lipoprotein (HDL), and use of antihypertensive medications to stratify CVD risk [32]. However, there is little data in patients less than 40 years old suggesting that FRS does not appropriately assess risk in younger patients, especially those with ED. It also lacks family history, fasting glucose level, serum creatinine, urinary albumin to creatinine ratio, and potentially, testosterone level that should be considered to estimate cardiovascular risk in men with ED. The Consensus continues to recommend the FRS as a useful starting tool to estimate subclinical atherosclerosis in men with ED [3]. However, it cautions the use of the FRS alone in men with ED aged 30–60 years without further investigation to other CVD risk factors [3]. ED should alert the physician to increased CVD risk.
The Consensus recommends risk assessments to all men who present with ED regardless of other symptoms [3]. A man with organic ED is considered at increased CVD risk until further workup suggests otherwise. ED can help to identify increased CVD risk in patients both with and without CVD symptoms or event history. The Panel recommends the risk assessments outlined in Table 22.2 [3], consistent with the 2010 ACCF/AHA guidelines [6], to identify men who may need further CVD workup. Therefore, a complete, noninvasive workup, managed by the primary care physician or cardiologist, is recommended, with invasive workup if necessary [33–36]. However, initial evaluation with more specialized tests may be warranted if symptoms suggest increased CVD risk [3].
Table 22.2
Recommendations for assessment of CVD in men with ED and no known CVD
Patient history | History, age, comorbid conditions (i.e., obesity, hypertension, dyslipidemia, prediabetes, OSA) |
Family history of premature atherothrombotic CVD (father aged <55 years or mother aged <65 years at cardiovascular event; ACCF/AHA class I, LOE B) | |
Lifestyle factors (i.e., diet, alcohol use, sedentary lifestyle, and smoking) | |
Physical exam | BP, waist circumference (WC), body mass index (BMI) |
Cardiac auscultation, carotid bruits, and palpation of femoral and pedal pulses | |
Fundal arterial changes | |
ED severity (using the International Index of Erectile Function score or Sexual Health Inventory of Men) and duration | |
Laboratorytesting | Fasting plasma glucose level |
Serum creatinine level (estimated glomerular filtration rate (eGFR)) | |
Albumin to creatinine ratio | |
Total testosterone (TT) level before 11 AM | |
Plasma lipid levels including total, low-density lipoprotein (LDL), and high-density lipoprotein cholesterol and triglyceride levels | |
High-sensitivity C-reactive protein (hsCRP) (ACCF/AHA class IIb, LOE B in asymptomatic, intermediate-risk men ≤50 years) [6] | |
Glycated hemoglobin (ACCF/AHA class IIb, LOE B in asymptomatic adults without diagnosis of DM) [6] | |
Urinary albumin excretion (ACCF/AHA class IIa, LOE B in asymptomatic adults with hypertension or DM; ACCF/AHA class IIb, LOE B in asymptomatic, intermediate-risk adults without hypertension or DM) [6] | |
Serum uric acid and lipoprotein-associated phospholipase A2 (ACCF/AHA class IIb, LOE B in asymptomatic, intermediate-risk adults) [6] | |
Invasive radiographic testing | |
Resting electrocardiogram (ACCF/AHA class IIa, LOE C in asymptomatic adults with hypertension or DM and ACCF/AHA class IIb, LOE C in asymptomatic adults without hypertension or DM) | |
Carotid intima-media thickness (CIMT) (ACCF/AHA class IIa, LOE B in asymptomatic, intermediate-risk adults) | |
Computed tomography for coronary artery calcium scoring (CACS) (ACCF/AHA class IIa, LOE B in asymptomatic, intermediate-risk men ≥40 years; ACCF/AHA class IIb, LOE B in low to intermediate risk [6–10 % 10-year risk] in men ≥40 years) | |
Ankle-brachial index (ABI) (ACCF/AHA class IIa, LOE B in asymptomatic, intermediate-risk adults) | |
CCTA (ACCF/AHA class III, LOE C in asymptomatic patients) | |
Pulse wave velocity (PWV) (ACCF/AHA class III, LOE C in asymptomatic patients) | |
Noninvasive assessment of endothelial function (i.e., brachial artery flow-mediated dilation) (ACCF/AHA class III, LOE B in asymptomatic adults) |
Given the significant evidence that ED is predictive of future CVD, the Panel recommends that lifestyle modifications will not only improve cardiovascular health but also improve erectile function as shown in Table 22.3 [3, 38].
Table 22.3
Lifestyle modifications shown to improve cardiovascular health
Modification | Clinical benefit |
|---|---|
Smoking cessation | 36 % decrease in mortality in CAD in a meta-analysis of prospective cohort studies [39] |
Regular dynamic exercise | |
Weight loss, dietary changes, and moderate alcohol consumption | Improved diet was shown to reduce CAD mortality by 36 % [44] |
Several of the recommendations are aimed at preventing progression and even helping to reverse atherosclerotic and other CVD [3]. DM is associated with a twofold increase in CVD [45] and is a CAD equivalent. A recent study of 4,883 men and women 65 years and older followed for 10 years suggested that 90 % of new cases of DM were preventable if patients were in the low-risk group five lifestyle factors shown in Table 22.4 [46].
Physical activity | Leisure-time activity |
Walking pace above the median | |
Diet | High-fiber intake |
High polyunsaturated to saturated fat ratio | |
Low trans-fat intake and low mean glycemic index | |
Smoking | Never smoker |
Former smoker more than 20 years ago or for fewer than 5 pack-years, | |
Alcohol use | Predominately light or moderate |
BMI and WC | BMI <25 |
WC <88 cm and <92 cm for women and men, respectively |
Waist circumference and waist to hip ratio are better predictors of cardiovascular outcomes than BMI [47, 48]. This is thought to be due to the secretion of excess free fatty acid, inflammatory cytokines, and reduced secretion of adiponectin from abdominal fat [49]. Chronic kidney disease also conveys significant cardiovascular morbidity and mortality. eGFR <60 ml/min and urinary albumin to creatinine ratios greater than 10 mg/g are associated with increased cardiovascular mortality independent of other traditional risk factors [50, 51].
Testosterone measurement has recently become controversial in men without symptoms of low testosterone. Testosterone plays important functions centrally and peripherally in erectile function in animal models [52–55]. This data has been consistent with clinical data. A single-center study of 1,050 men with new evaluation for sexual dysfunction found that 36 % had hypogonadism [56]. A meta-analysis of 7,000 men with ED in nine studies reported serum testosterone levels <300 ng/dL in 12 %. Hypogonadism is a potential cause of ED [57, 58], and testosterone replacement therapy (TRT) has been shown to improve response [58]. Recently, several large epidemiologic studies have associated low testosterone levels with increased all-cause and cardiovascular mortality shown in Table 22.5 [59–67]. However, caution should be advised, as there is no defined lower limit of normal TT.
Table 22.5
Low testosterone levels and increased mortality rates in recent publications with populations greater than 500 men
Reference | HR (95 % CI) | Study design | Men (no.) | Avg follow-up (year) | Mortality |
|---|---|---|---|---|---|
Shores et al. [59] | 1.88 (1.34–2.63) | Retrospective | 858 | 8.0 | All-cause |
Laughlinet al. [60] | 1.40 (1.14–1.71) | Prospective | 794 | 20.0 | All-cause |
1.38 (1.02–1.85) | CVD | ||||
Khawet al. [61] | 2.29 (1.60–3.26) | Prospective | 2,314 of 11,606 | 10.0 | All-cause and CVD |
Haringet al. [62] | 2.32 (1.38–3.89) | Prospective | 1,954 | 7.2 | All-cause |
2.56 (1.15–6.52) | CVD | ||||
Malkinet al. [63] | 2.27 (1.45–3.60) | Prospective | 930 | 6.9 | All-cause in men with CAD |
Tivesten et al. [64] | 1.65 (1.29–2.12) | Prospective | 3,014 | 4.5 | All-cause |
Menke et al. [65] | 1.43 (1.09–1.87) | Prospective | 1,114 | 9.0 | All-cause |
Vikan et al. [66] | 1.24 (1.01–1.54) | Prospective | 1,568 | 11.2 | All-cause |
Corona et al. [67] | 7.1 (1.8–28.6) | Prospective | 1,687 | 4.3 | CVD |
Additionally, a 2010 meta-analysis of cohort studies in middle-aged men showed no association between endogenous TT levels and CVD risk in middle-aged men [68]. A more recent meta-analysis of 49 cross-sectional studies showed increased CVD in low TT levels and high estradiol levels [69]. However, a separate meta-analysis of 19 studies showed that low TT does not correlate with CVD in healthy men <70 years [68]. The same study, did however, show that low testosterone predicts increased risk for CVD and mortality in elderly men [68]. Nonetheless, the authors cautioned that low TT may be a marker of poor health, rather than testosterone being protective of CVD. This idea is consistent with studies that androgen deficiency is associated with insulin resistance, DM II, metabolic syndrome, and increased deposition of visceral fat [70–73]. The TIMES2 (Testosterone Replacement in Hypogonadal Men With Either Metabolic Syndrome or Type 2 DM) study, a randomized, placebo-controlled study of 6–12 months of transdermal TRT vs. placebo in hypogonadal men with DM II, resulted in improved insulin resistance and glycemic control (ACCF/AHA class Ib) [74]. Additionally, a meta-analysis of five other randomized controlled trials with mean follow-up of 58 weeks showed TRT to be associated with a significant reduction in glucose homeostasis model assessment of insulin resistance index, triglycerides, and WC and an increase in HDL levels [75]. Nonetheless, randomized controlled trials for TRT risks and benefits are needed to establish CVD risk and mortality.
The American College of Physicians recommends only testing for testosterone levels in men with ED when accompanied by the presence of other symptoms (decreased libido, decreased spontaneous erection) or other physical exam findings (testicular mass or muscle atrophy) [76]. Conversely, the Consensus recommends CVD risk and testosterone levels be measured in all men diagnosed with organic ED [3], especially for those who have failed PDE5 inhibitor therapy [6], consistent with British Society for Sexual Medicine [77], International Society for Sexual Medicine [78], Endocrine Society [79], and a combined proposal from the International Society of Andrology, International Society for the Study of the Aging Male, European Association of Urology European Academy of Andrology, and the American Society of Andrology [80] as outlined in Fig. 22.1 [81].
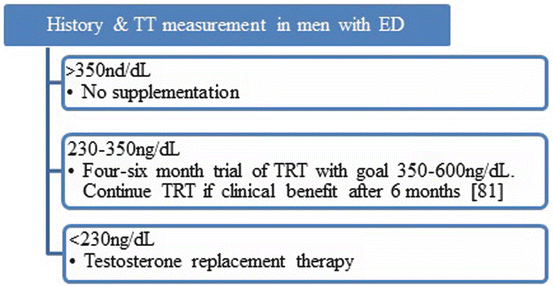

Fig. 22.1
TRT algorithm in men with ED. Use caution in those with congestive heart failure due to fluid retention. Men >70 years and those with multiple comorbidities should be treated with easily titratable TRT such as gel, spray, or patch for rapid removal should adverse effects develop. Baseline prostate-specific antigen and hematocrit testing required every 6 months
High sensitivity C reactive protein (hsCRP) is an independent predictor of coronary events after adjustment of traditional risk factors [6, 82–84] (i.e., age, total cholesterol, HDL, smoking, BMI, DM, hypertension, exercise level, and family history of CAD). Consequently, the Centers for Disease Control and Prevention and AHA recommended measurement of the hsCRP in addition to global risk prediction, especially those with intermediate risk [85]. The hsCRP level was also shown to improve the predictive value of the FRS in high-risk patients for CAD [86]. Similar evidence was also noted in a meta-analysis of 54 long-term prospective studies without history of vascular disease where high CRP was associated with increased risk of CAD, cerebrovascular disease, vascular mortality, and nonvascular mortality [87]. Additionally, there is evidence that hsCRP correctly reclassifies patients from intermediate-risk CVD to low or high risk [88].
Serum uric acid levels in asymptomatic patients are not specifically addressed in the 2010 ACCF/AHA guidelines [6]; however, the Consensus recommends serum uric acid measurement due to recent evidence that increased levels are predictive of increased cardiovascular risk [89].
A recent analysis from the Atherosclerosis Risk in Communities Study found that the addition of glycated hemoglobin to prediction models with traditional risk factors improved CAD risk prediction in nondiabetic patients with no previous history of CVD [90]. A meta-analysis of 26 cohort studies showed that microalbuminuria was associated with a 50 % increased risk of CAD. Macroalbuminuria more than doubled CAD risk [91]. However, overall there is inconsistency regarding adding glycated hemoglobin and urinary albumin to traditional risk factors in reassessing CVD risk.
Lipoprotein-associated phospholipase A2 levels were shown to be independent predictors of CVD in healthy adults after adjustment for hsCRP and standard risk factors [92–95]. Measurement of lipoprotein-associated phospholipase A2 is consistent with the 2010 ACCF/AHA guidelines for intermediate-risk adults [6].
EST may prove particularly beneficial to evaluate silent CAD risk in patients with ED and DM. In patients with DM II, 33.8 % of men with silent CAD had ED, while only 4.7 % of men had ED without silent CAD [10]. Chemical stress testing (with dipyrimadole or adenosine with nuclear imaging) is appropriate for patients who cannot undergo EST due to disabling condition [3]. If the baseline EKG makes EST unequivocal, the Panel recommends referral to a cardiologist [3].
The 2010 ACCF/AHA guidelines state that it is reasonable to perform CIMT, CACS, and ABI during CVD assessment of intermediate-risk patients [6]. This was further supported by the Society for Heart Attack Prevention and Eradication task force that stated all asymptomatic men 45–75 years and women 55–75 years who do not have very-low-risk characteristics or a documented history of CVD be evaluated with CACS or CIMT for subclinical CAD [96]. Analysis from the Atherosclerosis Risk in Communities Study, CIMT, and plaque detection with ultrasound added to traditional risk factors improved CAD detection [97]. The Multi-Ethnic Study of Atherosclerosis showed that CACS has better predictive value of CVD than CIMT [98]. A meta-analysis of 16 population-based cohort studies showed that ABI ≤0.90 was associated with twice the 10-year overall mortality, cardiovascular mortality, and cardiovascular events than each FRS category [99]. Recent meta-analysis of 17 longitudinal studies with 7.7-year average follow-up found that patients with high aortic PWV were also at higher risk for overall mortality, cardiovascular mortality, and cardiovascular events [100].
Although endothelial dysfunction has yet to be thoroughly studied in men with ED, endothelial dysfunction was found to be an independent predictor of cardiac death, MI, revascularization, or cardiac hospitalization in symptomatic outpatients over 7-year follow-up [101]. Additionally, brachial artery endothelial function assessed by flow-mediated dilation [102, 103] or in the forearm microvasculature assessed by Doppler flow [104] predicted poor cardiovascular outcome independent of the FRS.
22.3 Management of ED in the Patient with Known CVD
Previous recommendations from the prior two Princeton Consensus Conferences stratified patients into low-, intermediate-, and high-risk categories, where risk is defined as the likelihood of morbidity and mortality while engaged in or shortly after sexual activity [1, 2]. A number of groups were stratified into different groups in the most recent Conference. Patients with New York Heart Association (NYHA) class II CHF are low risk rather than intermediate risk, NYHA class III CHF are intermediate risk rather than high risk, and patients with mild, stable angina, and those with past MI (>6–8 weeks) are intermediate risk rather than low risk.
As discussed previously, a sexual inquiry into all men with ED should be undertaken to identify potential cardiovascular risk factors and incorporate lifestyle and therapeutic interventions as needed [3]. Exercise tolerance should be evaluated in all patients with ED [105]. A recent meta-analysis of ten studies showed that episodic activity was associated with an increased risk of MI and sudden cardiac death (SCD). However, this effect was attenuated in those with high habitual levels of activity. For every hour per week exposed in physical activity, the relative risk for MI and SCD was decreased by 45 % and 30 %, respectively [106]. This may aid in physicians to estimate cardiovascular risk associated with sexual activity in patients. Patients are placed in appropriate groups for treatment based on symptoms and recent cardiovascular events with appropriate treatment regimens outlined in Fig. 22.2 [3].
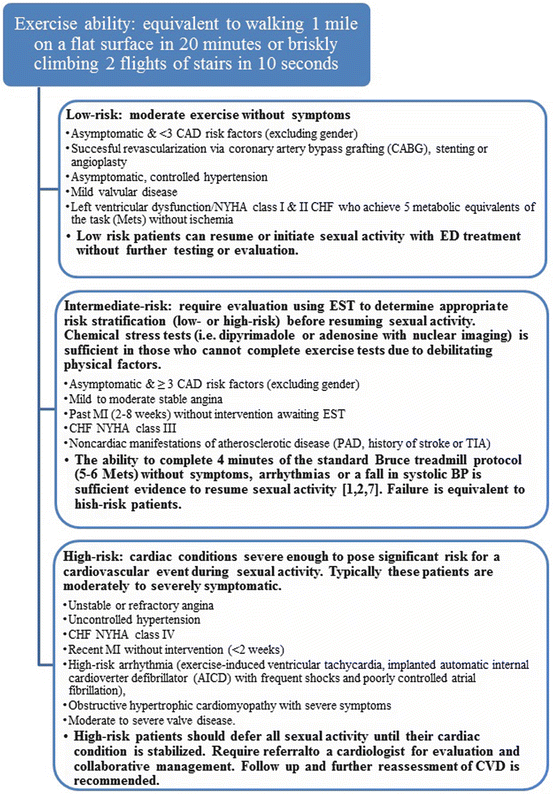

Fig. 22.2
Management of men with ED and known CVD
Interventions for the management of ED should not compromise cardiovascular function. The second Princeton Recommendations highlighted the pharmacologic treatment of ED [2]. PDE5 inhibitors have been safely shown to improve ED as the first-line treatment without new significant cardiovascular events in recent analysis of placebo-controlled and postmarketing surveillance data [107, 108]. It is important to educate patients on the interaction between organic nitrates, used for angina treatment, and PDE5 inhibitors. Due to the possibility of marked systemic hypotension, organic nitrates and PDE5 inhibitors are absolutely contraindicated [109–111]. If angina should develop with a PDE5 inhibitor present, at least 24 h for a short-acting PDE5 inhibitor (sildenafil, vardenafil) must elapse [112] prior to nitrate therapy, and at least 48 h must elapse for a long-acting PDE5 inhibitor (tadalafil) [113].
Alpha-blockers have varying degrees of interaction with PDE5 inhibitors that warrants starting at low dose of alpha-blockers until patients can adjust [2, 114]. Vardenafil has increased QTc by 6–9 ms and is not recommended in men taking type 1A or type 3 antiarrhythmic agents or men with known congenital prolonged QT interval [109]. The beta-blocker nebivolol is less likely to cause ED than other beta-blockers due to direct vasodilating properties [115–117]. Angiotensin receptor blockers have also been shown to less likely cause ED than diuretics [118, 119]. Additionally, statins have been reported to improve erectile function in men both with and without PDE5 inhibitors [120–123]. However, recent data showed that men reported new onset ED in 22 % of 93 high-risk men after 6 months of statin use [124]. Overall, there is a lack of placebo-controlled studies on erectile function in men taking medications to treat CVD.
In addition to their well-known treatment of ED, studies have actually shown a possible role for PDE5 inhibitors in the management of hypertension [125–128] and endothelial dysfunction [129–131] in patients at risk for CVD. This is not entirely surprising given their initial design for the treatment of pulmonary hypertension. In addition to PDE5 inhibitors, TRT should be incorporated for men as discussed previously. This can be incorporated initially or after PDE5 failure [58, 132]. Other nonpharmacologic approaches [2] such as exercise, weight loss [133, 134], and acknowledging partner and relationship factors [135–138] should be incorporated at all points.
Due to the evidence that suggests there is a window where ED presents prior to symptomatic CAD, ED may serve as a valuable identifier for men who should undergo a more extensive cardiovascular risk assessment [3]. This workup should be performed prior to resuming sexual activity with appropriate interventions as needed. A patient’s cardiovascular health must be properly evaluated and optimized prior to beginning therapy for ED as well. Lifestyle modifications are beneficial not only to improving cardiovascular health but also erectile function. The Princeton Consensus Panel recommends a collaborative, multidisciplinary approach to the management of all men with ED with primary care, cardiologic, endocrine, and urologic specialists [3].
References
1.
DeBusk R, Drory Y, Goldstein I et al (2000) Management of sexual dysfunction in patients with cardiovascular disease: recommendations of The Princeton Consensus Panel. Am J Cardiol 86(2):175–181PubMed
2.
Kostis JB, Jackson G, Rosen R et al (2005) Sexual dysfunction and cardiac risk (the Second Princeton Consensus Conference). Am J Cardiol 96(2):313–321PubMed
3.
Nehra A, Jackson G, Miner M et al (2012) The Princeton III consensus recommendations for the management of erectile dysfunction and cardiovascular disease. Mayo Clin Proc 87(8):766–778PubMedCentralPubMed
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


