Fig. 12.1
Effects on cardiovascular and renal function due to reduced sympathetic and increased parasympathetic drive resulting from baroreflex activation. After Floras [22]
The buffering action of the baroreflex on the circulation is measured by baroreflex sensitivity (BRS), defined as the change in duration of the cardiac cycle per unit change in BP. In the case of important cardiovascular pathophysiologies such as HF and hypertension, BRS is depressed; thus, the baroreflex is less active than normal in regulating cardiovascular performance [28, 29]. While this is an interesting observation from the standpoint of mechanism, it is also of clinical consequence: depressed BRS has been linked to adverse outcomes in HF [39, 46]. Presumably, the link is mediated by the increased sympathetic and decreased parasympathetic nerve traffic which accompany reduced BRS. The cardiovascular implications of these changes include elevated heart rate and cardiac automaticity, increased arterial resistance and stiffness, decreased natriuresis, and reduced splanchnic blood flow leading to increased blood volume and pressures in the pulmonary circuit and increased myocardial oxygen demand. Clinically, these effects translate to an environment conducive to arrhythmogenesis and worsening HF culminating in decompensation and circulatory failure. Importantly, reduced BRS appears to be driven at the receptor level rather than centrally [44], suggesting the premise that interventions which potentiate afferent carotid sinus nerve activity may improve BRS and associated outcomes.
Concordantly, direct assessment of efferent traffic has confirmed that therapies which counteract the effects of reduced BRS improve outcome in HFrEF. Muscle sympathetic nerve activity (MSNA), measured by microelectrodes percutaneously introduced into the peroneal nerve, provides a direct assessment of efferent traffic which parallels the activity of cardiac, renal, and other sympathetic nerves. MSNA is chronically decreased by treatments which indirectly affect sympathetic activity such as angiotensin-converting enzyme inhibitors, statins, and cardiac resynchronization therapy [27, 29, 30]. Beta-blocker therapy, which also improves outcome in HF but does not necessarily reduce sympathetic activity [7], interferes with efferent sympathetic activity by blocking receptors for norepinephrine while not antagonizing sympathetic cotransmitters including neuropeptide Y and adenosine triphosphate. In contrast, a therapy which produces indiscriminate sympatholysis worsens outcome in HF: the central alpha-agonist moxonidine produced excess mortality compared to placebo in the MOXCON trial [16, 21].
Given the link between reduced BRS, increased sympathetic tone, and adverse outcome in HF, it is logical to consider exogenous means of activating the baroreflex as a therapy. Although it was not conceived as such, medicinal use of digitalis is the most long-standing and widely used therapy. Digitalis acts, at least in part, by sensitizing baroreceptors so that BRS is increased [20]. Results from the DIG trial have shown that digitalis improves outcome in both HFrEF and HFpEF [2, 3]. Unfortunately, digitalis is difficult to apply practically due to its narrow therapeutic window and toxic effects from overdose [47], problems that can be exacerbated by diuretic therapy and renal dysfunction which are both common in HF.
Nonpharmacologic means have also been used to activate the baroreflex in HF. The technique of carotid massage has been known for centuries and remains in wide use today as a means of diagnosing and acutely treating conduction abnormalities and supraventricular arrhythmias. In a case series reported in the 1950s, it was also incorporated into a protocol for the treatment of acute decompensated HF. Patients improved when subjected to massage-induced baroreflex activation, with symptoms including hypertension, elevated heart rate, pulmonary edema, and dyspnea quickly resolving [4].
With the advent of active implantable device technology, a system was developed to transiently activate the baroreflex through direct stimulation of the carotid sinus nerve. The device was used in patients with refractory angina and symptom-limited functional capacity. Acute activation of the device in clinical trials was demonstrated to increase functional capacity [13] and reduce cardiac filling pressures [19]. Technological limitations and the development of bypass grafting precluded widespread use of the system, but the potential benefits had been demonstrated.
12.3 Baroreflex Activation Therapy (BAT)
Technological improvements in electronics and battery technology, coupled with continued poor outcome of HF patients, motivated the development of a new approach to chronically activate the baroreflex. The BarostimTM neo TM system (Fig. 12.2) consists of an implanted pulse generator with a form factor similar to a contemporary implanted defibrillator coupled to a carotid sinus lead. Implant of the electrode directly on the carotid sinus provides for chronic activation of the baroreflex without the need to surgically isolate the carotid sinus nerve. Improved device technology allows the system to continuously excite carotid baroreceptors for 3–4 years using a single battery. The system is implanted unilaterally, typically on the right side. The lead is affixed directly to the carotid sinus with sutures and subcutaneously tunneled to the pectoral region where the pulse generator is implanted. Right-sided implant is motivated by the fact that patients frequently have cardiac rhythm management devices preferentially placed on the left side and by clinical experience demonstrating that full therapeutic efficacy may be obtained with unilateral, right-sided implant [18]. System implant time averages approximately 90 min for new implanters and is often less than 60 min among experienced implanters. The system safety profile is comparable to a pacemaker [36].
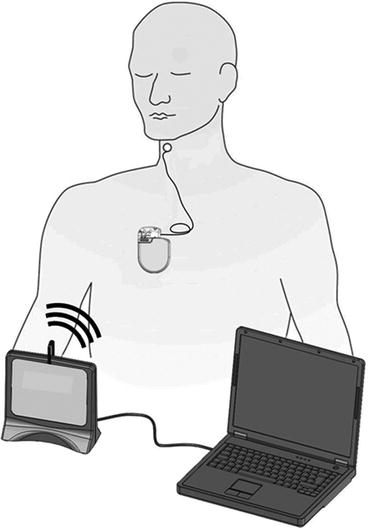

Fig. 12.2
Schematic depiction of the implantable baroreflex activation therapy system. A pulse generator similar in size to an implanted defibrillator delivers electrical stimulation via a lead implanted on the arterial surface in the vicinity of the carotid sinus. The system is controlled by a laptop computer running custom software. Information is exchanged between the computer and pulse generator through a radiofrequency communication link
The implanted system is controlled by a programmer consisting of a laptop computer, custom software, and an antenna module. Programming and device status information is transmitted wirelessly via a radiofrequency link with a range of several meters. Therapy intensity is typically up-titrated over the course of the first 3 months using a standardized procedure and is then adapted as necessary according to patient needs. Programmable parameters include pulse amplitude, pulse width and pulse frequency, as well as duty cycle and the possibility of applying different therapy regimens based on time of day to accommodate diurnal rhythms.
12.4 Basic Science Supporting the Use of BAT in HF
An extensive literature exists on the physiologic effects of the baroreflex. It is too vast to be adequately described here, but an overview relevant to BAT in HF has been produced by Georgakopoulos and colleagues [26]. This review will focus on studies of the baroreflex conducted specifically with BAT.
12.4.1 Animal Studies
Acute studies of BAT in animal models have demonstrated sustained salutary effects on cardiac pump efficiency, arteries, veins, and myocardial automaticity. BAT intervention in normal canines significantly increases stroke volume and reduces heart rate while preserving cardiac output [23]. This is achieved by reducing systemic vascular resistance and arterial stiffness. Despite accompanying acute reductions in BP, average renal blood flow is unchanged while flow pulsatility increases [51]. The reduced myocardial oxygen demand, increased cardiac reserve, and maintained perfusion would be expected to improve functional capacity and protect against arrhythmogenic effects of myocardial ischemia in HF. Decreased pressure transmission with maintained perfusion through the renal artery may guard against increases in renin and help preserve renal function, thereby lessening the chances of developing cardiorenal syndrome.
In addition to arterial effects, venous capacitance is modulated by BAT. Acute BAT induces sustained transfer of blood volume from the arterial to the venous circulation in normal dogs [14]. At equal reductions in BP, BAT produces a comparable capacitance change but a more modest increase in arterial conductance as compared to sodium nitroprusside. BAT also reduces heart rate while nitroprusside increases it. Volume transfer in HF results in reduced cardiac filling pressures, thereby improving ejection as well as filling. Submaximal change in arterial conductance and reduced heart rate suggest that BAT provides greater reserve to adapt to physiologic need.
BAT also exerts electrophysiologic effects on the myocardium. In studies of normal dogs subjected to ischemia and infarction, BAT increased effective refractory period and action potential duration while reducing incidence of ventricular arrhythmia [42, 43]. In addition, BAT reduced cardiac sympathetic nerve traffic and the heart rate variability (HRV) low-frequency to high-frequency power ratio (LF/HF), indicating suppression of sympathetic nerve activity associated with ischemia and infarction.
The foregoing results are encouraging but raise an important question: if the baroreflex is constantly activated by BAT, has the ability of the cardiovascular system to adapt to physiologic need been compromised (i.e., is it possible that BAT lowers BRS)? Both acute and chronic studies indicate that the answer is no. In acute studies of rats, Avolio and colleagues demonstrated that reflex responses to bolus infusions of phenylephrine and sodium nitroprusside were not impaired by BAT [38]. Rather, the reduced BP driven by BAT acted as a new set point around which the reflex responses were superimposed. Calculations revealed that BAT actually increased BRS. Similarly, Iliescu reported that chronic BAT administered to obese hypertensive canines chronically increased BRS [37]. Thus, the acute hemodynamic improvements of BAT, coupled with improved adaptability of the cardiovascular system, would be expected to improve chronic HF.
Consistent with these expectations, chronic studies of BAT in two canine models of chronic HF demonstrated significant benefit. In a rapid pacing model, BAT doubled the duration of survival while reducing filling pressures and plasma norepinephrine [54]. In a microembolization HFrEF model, BAT increased ejection fraction, reduced cardiac fibrosis, and normalized myocyte intracellular factors associated with beta receptor signaling, a characteristic derangement in human HF [49]. A second study of the microembolization model demonstrated a protective effect of BAT against induction of ventricular arrhythmia lasting at least 6 months that was reversed 6 weeks after BAT withdrawal [50].
In summary, preclinical evaluations of BAT illustrate important mechanistic pathways through which BAT can benefit HF, including greater cardiac pump efficiency, reduced systemic vascular resistance, increased venous capacitance, as well as protection against myocardial ischemia, ventricular arrhythmia, and declining renal function. The effects are mediated through tonic reductions in sympathetic activity and increases in parasympathetic activity. Most importantly, BAT improves survival.
12.4.2 Human Studies
Mechanisms of BAT have also been explored in the clinical environment. Investigator-initiated substudies of feasibility and commercial hypertension patients have afforded opportunities to confirm that mechanisms observed in animal studies translate to patients. A study of HRV from serial Holter recordings found BAT chronically increased LF/HF ratio and decreased LF power, indicating reduced sympathetic activity and increased parasympathetic activity [51]. Moreover, the study found that BAT increased turbulence onset associated with premature ventricular contractions, implying that BAT increased BRS [40]. These findings were further validated by acute measurement of MSNA, which demonstrated a direct on-off relationship between initiation of BAT and reduction of efferent sympathetic traffic [34]. Pulse wave analysis of concomitantly acquired continuous BP exhibited improved ventricular-vascular coupling with reduction of wasted left ventricular external work and improved myocardial perfusion with an increase in subendocardial viability ratio [35].
Chronic hemodynamic improvements have also been observed clinically with BAT. From serial echocardiography and BP measurement, BAT was shown to reduce myocardial wall stress [24] and arterial elastance [25], indicating reduced filling pressures associated with diminished load. Importantly, cardiac contractility was not affected. Direct measurement of cardiac hemodynamics was also conducted in one patient [51]. Consistent with animal studies, BAT substantially increased stroke volume and reduced heart rate while maintaining cardiac output. Systemic vascular resistance and arterial stiffness diminished. Afterload was reduced and diastolic filling improved as assessed by peak filling rate despite reduced left atrial pressure, suggesting restoration of the ability of the left ventricle to enhance early filling. Taken as a whole, the clinical studies of mechanism strongly suggest that benefits of BAT observed in animals translate directly to the clinical environment.
12.4.3 BAT and Blood Pressure in HF
While results from basic science provide encouragement that BAT can benefit HF patients, it is appropriate to reflect on the implications of validating mechanisms of action in hypertensive patients. Hemodynamic and neural changes were accompanied by reductions in BP. For patients with HFpEF, this is generally not a concern, as many are hypertensive and contractile function is unimpaired. However, HFrEF patients are typically normotensive to hypotensive and with diminished cardiac contractility. Moreover, low BP has been associated with adverse outcomes in these patients [5].
In this context, it is important to consider the primary means by which BAT reduces BP, namely, through reduced arterial resistance and stiffness. The greater the contractile function of the heart, the more BP is diminished per unit reduction in arterial resistance [11]. Therefore, it is expected that BAT will reduce BP substantially in hypertensive patients but should have only modest effects in HFrEF patients. In some patients, contractile function may be so compromised that no change in BP is detectable at all. Because BAT does not affect cardiac contractility, acute changes will be dictated solely by reduced arterial smooth muscle tone. On a chronic basis, it is possible that BAT may even increase BP, for as the heart is chronically unloaded, pump function may recover. This could be further enhanced through reduced left atrial pressure as well as increased BRS and exercise tolerance.
12.5 Clinical Trials of BAT in HF
With thoroughly demonstrated mechanisms of action and successful proof-of-concept animal studies, the rationale for clinical application of BAT in HF is clear. If clinical results verify that BAT mechanisms of action are operative in HF, one would reasonably expect BAT to improve outcome for the reasons cited above.
12.5.1 First-in-Man Experience with BAT in HFrEF
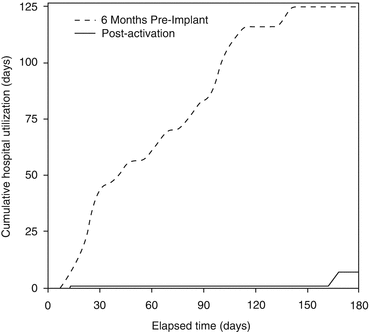
An open-label, single-center feasibility study of BAT in HFrEF was undertaken to confirm the translation of basic science results to the clinical environment [31]. Patients were required to have NYHA class III HF, EF ≤ 40 %, 6-min hall walk distance (6MHWd) between 150 and 450 m while receiving optimal, stable medical therapy. Eleven patients were enrolled in the study and followed for 6 months. MSNA, clinical variables, and echocardiograms were collected at baseline, 3 months, and 6 months. MSNA was also recorded at 1 month. Results demonstrated a progressive decline in MSNA consistent with BAT up-titration, reaching a 30 % reduction at 6 months (Fig. 12.3). Concurrent with reduced MSNA were clinically significant improvements in BRS, NYHA class, 6-min walk distance, LVEF, and quality of life as assessed with the Minnesota Living with HF Questionnaire (QOL). BP and renal function were unchanged from baseline. It was also observed that the cumulative number of HF hospitalization days was dramatically reduced in the 6 months following activation as compared to the 6 months prior to enrollment (Fig. 12.4). It has been recently reported that the clinical benefits can be observed at the long-term follow up of 21 months [32]. The long-term chronic BAT-dependent reductions in sympathetic activity is accompanied, in HFrEF, by an improvement in general clinical presentation, quality of life and functional capacity thus inducing an improvement in outcome.
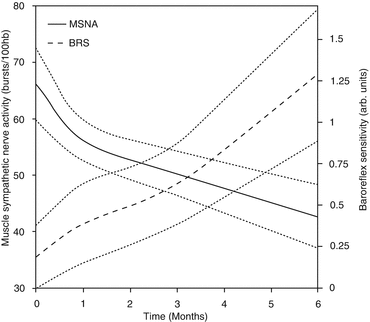

Fig. 12.3
Decreasing MSNA and increasing BRS trends observed in a study of 11 HFrEF patients receiving chronic BAT [31], as illustrated with exponential regression model fits paired with 95 % confidence intervals