, Marilyn J. Siegel2, Tomasz Miszalski-Jamka3, 4 and Robert Pelberg1
(1)
The Christ Hospital Heart and Vascular Center of Greater Cincinnati, The Lindner Center for Research and Education, Cincinnati, OH, USA
(2)
Mallinckrodt Institute of Radiology, Washington University School of Medicine, St. Louis, Missouri, USA
(3)
Department of Clinical Radiology and Imaging Diagnostics, 4th Military Hospital, Wrocław, Poland
(4)
Center for Diagnosis Prevention and Telemedicine, John Paul II Hospital, Kraków, Poland
Abstract
Ebstein anomaly is a myopathy of the right ventricle that results in variable degrees of failure of delamination of the valve leaflets, leading to tricuspid valve regurgitation and right ventricular dysfunction. The classic pathology is apical (downward) displacement of the tricuspid valve leaflets (most frequently septal followed by posterior and anterior leaflets) with abnormal adherence of the leaflets to the underlying myocardium.
12.1 Ebstein Anomaly
Ebstein anomaly is a defect of the right ventricle that results in variable degrees of failure of delamination of the valve leaflets, leading to tricuspid valve regurgitation and right ventricular dysfunction. The classic pathology is apical (downward) displacement of the tricuspid valve leaflets (most frequently septal followed by posterior and anterior leaflets) with abnormal adherence of the leaflets to the underlying myocardium.
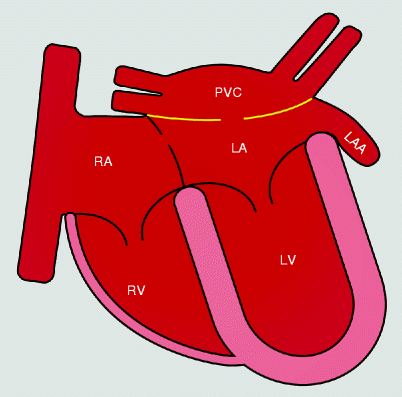
The major morphologic features are apical displacement of the septal leaflet ≥8 mm/m2, dilatation of the “atrialized” portion of the right ventricle with variable degrees of thinning of the free wall of the right ventricle [1]. Dilatation of the right atrioventricular junction (true tricuspid annulus) is also seen [1]. See Fig. 12.1. Incompetence of the deformed tricuspid valve and the functional impairment of the right ventricle result in an impediment to forward flow of blood through the right side of the heart. Typically, there are other anatomic abnormalities including a large, redundant anterior leaflet with fenestration and varying degrees of tethering to the right ventricular wall. In most cases there is a patent foramen ovale or secundum atrial septal defect, resulting in a right-to-left shunt due to elevated right-sided pressures.
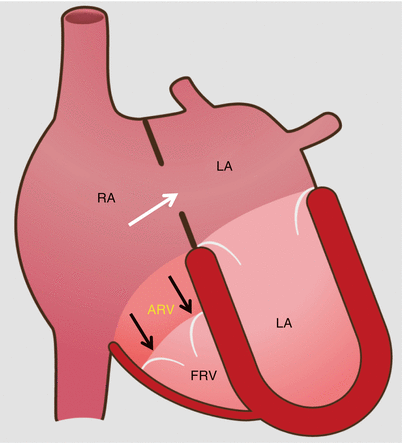

Fig. 12.1
Schematic drawing of Ebstein anomaly. The tricuspid valve is displaced toward the apex of the right ventricle with subsequent “atrialization” of a portion of the morphologic right ventricle, which is contiguous with the enlarged right atrium. The functional right ventricle is small in size. Note the secundum atrial septal defect (white arrow) which is commonly associated with Ebstein anomaly. The black arrows point to the apically displaced tricuspid valve. RA right atrium, ARV atrialized right ventricle, FRV functional right ventricle, LA left atrium, LV left ventricle
According to Carpentier’s classification, Ebstein anomaly can be categorized as follows: type A: adequate RV volume; type B: large atrialized segment of the RV and mobile anterior leaflet; type C: restricted movement of anterior leaflet which may cause infundibular obstruction; type D: near-complete atrialization of the RV (Uhl’s syndrome) [2].
12.1.1 Clinical Features
The downward displacement of the septal tricuspid valve leaflet is associated with discontinuity of the central fibrous body and septal atrioventricular ring, resulting in accessory atrioventricular connections and ventricular pre-excitation making the patient at risk of sudden death. Atrial arrhythmias are also common because of the enlargement of the atria.
Ebstein anomaly usually presents in neonates or infants with findings of congestive heart failure or profound cyanosis. Occasionally, however, the diagnosis is not made until late in life and presentations as late as the eighth decade have been reported [3]. Operative management of adult patients with Ebstein anomaly includes tricuspid valve repair or replacement, closure of the atrial septal defect (if present), as well as bidirectional cavopulmonary shunt, which is useful in patients with severe right ventricular dilatation and/or dysfunction. Late complications in palliated as well as unoperated patients include right and left ventricular failure, atrial and ventricular arrhythmias, and sudden cardiac death.
12.1.2 Cardiac Computed Tomography (CT) in the Evaluation of Ebstein Anomaly
Ebstein anomaly is easily detected by CT [4–6]. Imaging findings include a large right atrium proper, a dilated atrialized right ventricle, a small true right ventricle along with apical displacement of the septal leaflet of the tricuspid valve, and a “sail-like” anterior tricuspid valve leaflet deformity (Fig. 12.2) [4–6]. The atrialized ventricular wall may be thinner than the distal functional right ventricle, and the heart may rotate posteriorly into the left hemithorax with bulging of the ventricles posteriorly. An atrial septal defect is an associated finding (Fig. 12.3). Cine CT can offer functional information in addition to anatomy when retrospective gating is employed.
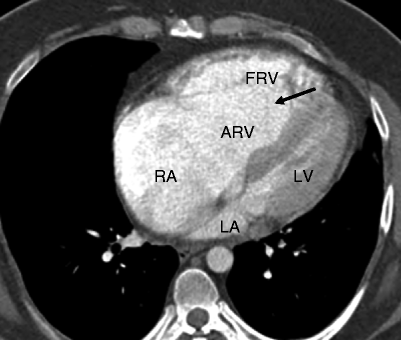
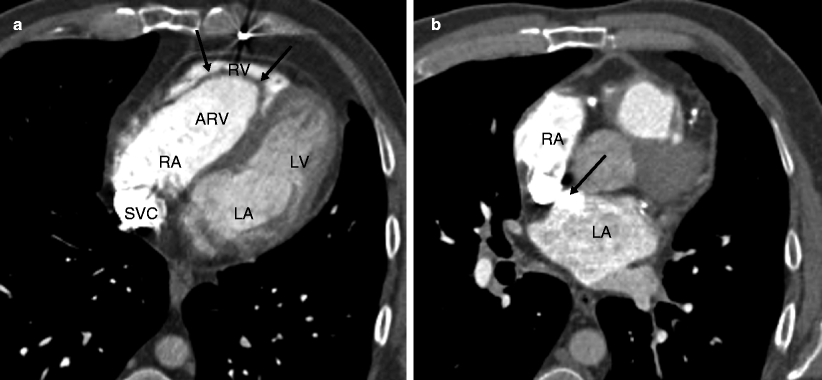

Fig. 12.2
Ebstein anomaly. An axial computed tomographic scan showing a dilated right atrium (RA), atrialized right ventricle (ARV), and a small functional right ventricle (FRV). Note the apical displacement of the septal tricuspid leaflet (black arrow). LA left atrium, LV left ventricle

Fig. 12.3
Ebstein anomaly. Panel (a) is an axial tomogram showing marked apical displacement of the septal tricuspid leaflet (arrows) resulting in atrialization of the right ventricle (ARV). Panel (b) is an axial cut at a more superior level demonstrating an atrial septal defect with contrast-enhanced blood (arrow) flowing into the left atrium (LA) from the right atrium (RA). SVC superior vena cava, RV right ventricle proper
12.2 Tricuspid Atresia
Tricuspid atresia is the third most common form of cyanotic congenital heart disease, with a prevalence of 0.3–3.7 % [7]. Tricuspid atresia is characterized by the absence of a tricuspid valve. There is no direct connection between the right atrium and right ventricle, resulting in a small or absent right ventricle that cannot adequately pump blood to the lungs (Fig. 12.4). Depending on the relationship with the great vessels, there are three types of tricuspid atresia. In type I, the great arteries are related normally. With type II, the great arteries are d-transposed, and in type III, the great arteries are l-transposed. These types are further subclassified according to the presence or absence of ventricular septal defects and pulmonary valve pathology [8, 9].
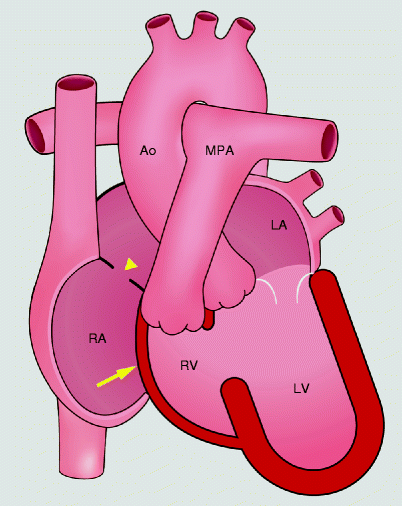

Fig. 12.4
Schematic drawings of tricuspid atresia. The tricuspid valve is absent which prevents antegrade flow into the right ventricle. The right ventricle is hypoplastic. An atrial septal defect supplies blood flow to the left heart. yellow arrow absent tricuspid valve, yellow arrowhead atrial septal defect, RA right atrium, RV right ventricle, LA left atrium, LV left ventricle, PA pulmonary artery, Ao aorta
Associated defects coexist in 15–20 % of patients, most frequently transposition of the great vessels and persistent left-sided superior vena cava [7]. Atrial septal defect is obligatory in those patients. Extracardiac associations may include a right-sided aortic arch and an absent spleen.
In patients with tricuspid atresia, venous blood returning to the right atrium can exit only through an interatrial communication. Because of the obligatory right-to-left shunt at the level of the atria, saturation of the left atrial blood is diminished. Additionally, intracardiac blood flow depends on coexisting pulmonary artery pathology. With coexistent pulmonary artery or pulmonary valve stenosis, pulmonary blood flow is reduced, resulting in worsening cyanosis. Pulmonary obstruction occurs most often in patients with tricuspid atresia and normal great artery anatomy. In the absence of pulmonary atresia or pulmonary stenosis, the volume of blood flowing to the lungs may be normal thus resulting in decreased cyanosis. Patients with d-transposed great arteries and tricuspid atresia typically have unobstructed pulmonary blood flow.
Tricuspid atresia patients ultimately develop left ventricular systolic dysfunction due to left ventricular volume overload related to the fact that venous blood must return to the left ventricle as well as to the presence of chronic hypoxemia. Left ventricular dysfunction may then lead to mitral annulus dilatation and mitral regurgitation, which further increases left ventricular volume overload.
12.2.1 Clinical Features
Tricuspid atresia is usually detected in infancy because of cyanosis, congestive heart failure, and growth retardation. Most patients with tricuspid atresia require some form of surgical treatment during the first year of life. Thus, most adults with tricuspid atresia have undergone surgical palliation. Since the primary lesion is not reparable, the basic features are still present in the palliated adult as are the findings of the palliative surgical procedure.
In neonates and infants, palliative or definitive surgery may be undertaken to connect the systemic circulation to the pulmonary circulation. Palliative surgery encompasses either a Blalock–Taussig shunt or a Glenn shunt (end-to-end anastomosis of the right pulmonary artery to the superior vena cava). Definitive surgery entails performing the total cavopulmonary Fontan procedure. The Fontan operation excludes the right ventricle through the formation of a right atrial-to- pulmonary artery connection or an extracardiac cavopulmonary anastomosis using a synthetic graft. Long-term complications of these palliative surgeries include right heart failure, mural thrombus, and dilatation of the cardiac/systemic veins that act as collateral vessels.
12.2.2 Cardiac Computed Tomography (CT) in the Evaluation of Tricuspic Atresia
CT has proven to be an excellent imaging modality to determine the anatomic features of tricuspid atresia [5, 6, 9, 10]. The classic finding is fatty tissue between the right atrium and the right ventricle in the expected location of the tricuspid valve and a small right ventricle (Fig. 12.5). The left ventricle is enlarged. Other features include atrial and/or ventricular septal defects (Fig. 12.6), transposition of the great arteries, and dilated inferior vena cava and hepatic veins due to passive congestion, mural thrombus, and collateral vessel formation. Postoperative CT can provide information on the patency of any shunts and on the size and morphology of the pulmonary arteries [11].
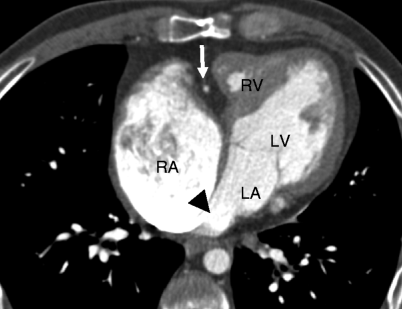
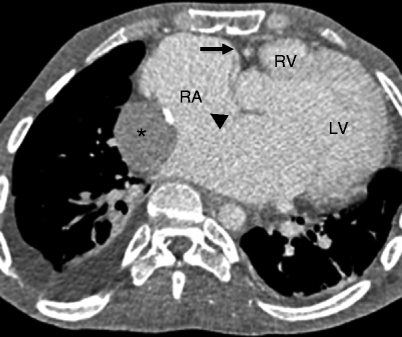

Fig. 12.5
Tricuspid atresia. An axial computed tomogram showing fat in the atrioventricular groove (arrow). The right atrium (RA) is enlarged and the right ventricle (RV) is hypoplastic. The black arrowhead points out the obligatory atrial septal defect. LV left ventricle, LA left atrium

Fig. 12.6
Tricuspid atresia with an atrial septal defect. This axial computed tomogram shows a fatty separation of the right atrium from the right ventricle (arrow), a hypoplastic right ventricle (RV), and enlarged right atrium (RA). A large atrial septal defect is noted (black arrowhead). Asterisk: extracardiac Fontan conduit. LV left ventricle
12.3 Congenital Mitral Inflow and Mitral Valve Abnormalities
12.3.1 Lesions Associated with Mitral Valve Obstruction
Cor Triatriatum Sinister
Cor triatriatum accounts for 0.1–0.4 % of all congenital cardiac anomalies [12]. It is a result of embryologic failure of the common pulmonary vein to become incorporated into the left atrium during the fifth week of embryologic development [13]. As a result, the left atrium is divided by a fibromuscular membrane into two chambers. The superior chamber receives blood from the pulmonary veins, and the inferior chamber is connected to the left atrial appendage and mitral valve. The two chambers communicate with each other through openings in the membrane (Figs. 12.7 and 12.8). Associated anomalies occur in 50–80 % of cases and include a patent foramen ovale, patent ductus arteriosus, atrial and ventricular septal defects, and persistent left-sided superior vena cava [12].