Atrial Septal Defect and Patent Foramen Ovale
Justin M. Dunn
Richard A. Krasuski
I. INTRODUCTION
A. Atrial septal defects (ASDs) constitute approximately 5% to 10% of congenital heart disease. Excluding bicuspid aortic valve and mitral valve prolapse, ASD is the most common form of congenital heart defect found among adults and is the most common acyanotic shunt lesion in adults as well.
B. Often, an atrial communication may go unrecognized into adulthood because the clinical symptoms and physical manifestations can be subtle.
C. Although survival into adulthood is the rule, the overall life expectancy is decreased in patients with an unrepaired ASD. Long-term exposure to chronic right heart volume overload can have deleterious effects, such as atrial arrhythmias, pulmonary vascular disease, and right heart failure. These clinical findings are directly related to patient age, with almost all patients becoming symptomatic by the fifth or sixth decade. The presence of an atrial communication is also a potential source of paradoxical embolus.
D. A patent foramen ovale (PFO) is a specific form of interatrial communication caused by incomplete closure of the foramen ovale after birth. PFOs are present in 25% to 30% of the general population. The prevalence of PFO in patients with cryptogenic stroke is approximately 40% to 50%.
E. Atrial septal aneurysms are congenital outpouchings of the atrial septum, near the fossa ovalis. These can perforate, resulting in an ASD with left-to-right shunting of blood. Atrial septal aneurysms can be detected in up to 10% of patients undergoing echocardiography and in up to 30% of patients with cryptogenic stroke, generally with a concomitant PFO.
II. ANATOMY AND EMBRYOLOGY.
The primitive atrium is first partitioned into right and left atria by growth of the septum primum—a thin, crescent-shaped membrane that grows from the roof of the primitive atrium toward the endocardial cushions located between the atria and ventricles. An atrial communication initially persists as the foramen primum, composed of the free edge of the septum primum and the endocardial cushions. Before closure of the foramen primum, fenestrations develop in the septum primum that coalesce to form the ostium secundum. As the septum primum then fuses with the endocardial cushions, the ostium secundum maintains a right-to-left atrial flow that is important in the fetal circulation. Failure of this fusion results in the development of a primum ASD. A second septum, the septum secundum, then forms to the right of the septum primum, growing toward the endocardial cushions and usually closing the ostium secundum. Failure to close the ostium secundum results in the formation of a secundum ASD.
The septum secundum forms an incomplete partition of the atria, leaving a foramen ovale (i.e., fossa ovalis). The remaining septum primum tissue on the left atrial (LA) side
becomes a flap valve, or valve of the foramen ovale, and allows for the continued rightto-left shunting in the fetal circulation. At birth, when LA pressure increases, the septum primum flap closes and eventually fuses to anatomically seal the atrial septum. A “true ASD” results from a deficiency in septal development or from resorption of atrial tissue, whereas a PFO results from failure of this septum primum flap to adequately seal the fossa ovalis. At autopsy, a “probe-patent” PFO remains in 25% to 30% of patients. During development, if there is overabundant or weakened septal tissue, the septum becomes very mobile. This can be visualized during echocardiography, and the degree of excursion can be measured. If the maximal excursion of the interatrial septum is 15 mm or more, this abnormality is called an atrial septal aneurysm. If the amount of septal excursion is < 15 mm, it is referred to as a redundant atrial septum.
becomes a flap valve, or valve of the foramen ovale, and allows for the continued rightto-left shunting in the fetal circulation. At birth, when LA pressure increases, the septum primum flap closes and eventually fuses to anatomically seal the atrial septum. A “true ASD” results from a deficiency in septal development or from resorption of atrial tissue, whereas a PFO results from failure of this septum primum flap to adequately seal the fossa ovalis. At autopsy, a “probe-patent” PFO remains in 25% to 30% of patients. During development, if there is overabundant or weakened septal tissue, the septum becomes very mobile. This can be visualized during echocardiography, and the degree of excursion can be measured. If the maximal excursion of the interatrial septum is 15 mm or more, this abnormality is called an atrial septal aneurysm. If the amount of septal excursion is < 15 mm, it is referred to as a redundant atrial septum.
ATRIAL SEPTAL DEFECTS
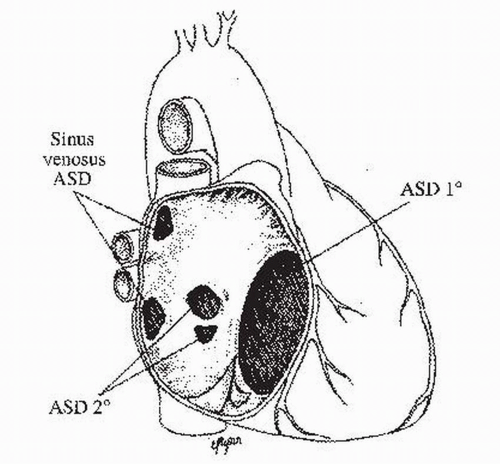
I. ASD TYPES (Fig. 29.1)
A. Ostium secundum defects or secundum ASDs constitute the most common type, accounting for 70% to 75% of ASDs. This defect, a true defect of the atrial septum, is located in the midportion of the atrial septum, within or including the fossa ovalis. Defects result from a deficient septum primum or an abnormally large foramen secundum. This type of ASD is two times more common in female patients. Isolated secundum ASD has been associated with mitral valve prolapse and other forms of congenital heart disease. It may also be associated with rheumatic mitral stenosis (i.e., Lutembacher syndrome).
B. Ostium primum defects or primum ASDs account for 15% to 20% of ASDs and are part of the spectrum of atrioventricular (AV) septal defects (also known as AV canal defects or endocardial cushion defects). These defects occur in the inferioranterior portion of the atrial septum and are frequently associated with a cleft in the anterior leaflet of the mitral valve, leading to varying degrees of mitral regurgitation. In their complete form, they include a large ventricular septal defect and a common
AV valve. Depending on the severity of dysfunction of the mitral valve, these patients may become symptomatic at a young age. This defect in the inlet septum is the most common ASD associated with Down’s syndrome.
AV valve. Depending on the severity of dysfunction of the mitral valve, these patients may become symptomatic at a young age. This defect in the inlet septum is the most common ASD associated with Down’s syndrome.
C. Sinoseptal defects constitute the remaining 5% to 10% of septal defects. Distinct from the true ASDs described previously, these lesions involve the portion of the atrial wall derived from the sinus venosus (i.e., there is no direct communication between the right and left atria). Sinus venosus defects are typically at the orifice of the superior vena cava (SVC) at the junction of the right atrium or, less frequently, in the region of the inferior vena cava (IVC). These sinus venosus defects are frequently associated with partial anomalous pulmonary venous drainage of the right pulmonary veins and require a high index of suspicion for diagnosis because they are generally not visualized by standard transthoracic echocardiography (TTE). Transesophageal echocardiography (TEE) is generally required for visualization in adults. Magnetic resonance imaging (MRI) or computed tomography may also be used for diagnosis. These defects should be considered in any patient with unexplained right atrial (RA) or right ventricular (RV) dilation. An uncommon sinoseptal defect is the partially or completely unroofed coronary sinus, which is located inferior and slightly anterior to the fossa ovalis. These defects are commonly associated with other forms of congenital heart disease, such as complete AV septal defect, or can be associated with an absence of coronary sinus and a left SVC that drains into the left atrium.
II. PATHOPHYSIOLOGY.
The magnitude and direction of the shunt through the ASD depend on the size of the defect as well as the diastolic filling properties of the ventricles. Any condition that causes reduced left ventricular (LV) compliance, such as LV hypertrophy or LV scar, or increased LA pressure, such as mitral stenosis, will increase the degree of left-to-right shunting. Conversely, conditions that cause reduced RV compliance, such as pulmonary hypertension or pulmonary stenosis, or increased RA pressure, such as tricuspid stenosis, will reduce the degree of left-to-right shunting and, in some instances, even lead to shunt reversal. In general, the ASD must be at least 10 mm in its greatest dimension to cause a significant shunt, although this can be hard to measure, as most ASDs are not circular. A left-to-right shunt is considered significant when the ratio of pulmonary-to-systemic blood flow, or shunt fraction (Qp/Qs), is > 1.5:1.0 or when right heart chamber dilation is present.
III. CLINICAL MANIFESTATIONS.
The clinical presentation of a patient with an ASD results from the effects of long-term left-to-right shunting and subsequent volume loading of the right heart. The age at which the symptoms occur is variable and does not necessarily depend on the size of the defect.
A. Exercise intolerance with fatigue and dyspnea may occur, but it is frequently not appreciated by the patient until after the defect has been closed. Late findings include supraventricular arrhythmias, such as atrial fibrillation or flutter, severe irreversible pulmonary vascular disease, and, eventually, right heart failure. Occasionally, a paradoxical embolus causing a stroke or transient ischemic attack (TIA) is the first clue to an ASD.
B. The physical findings may include a hyperdynamic cardiac impulse, the characteristic widely or fixed split second heart sound, and a soft systolic murmur at the second left intercostal space due to increased flow across the pulmonary valve. If the shunt is more than a shunt fraction (Qp/Qs) of 2.5:1, there may be a diastolic murmur secondary to increased flow across the tricuspid valve. A loud P2 component of the second heart sound indicates the presence of pulmonary hypertension, which can affect up to 20% of patients; if cyanosis is present, this generally suggests advanced pulmonary hypertension with reversal of shunt flow (Eisenmenger syndrome). An important clue to the presence of Eisenmenger syndrome is an oxygen saturation that does not significantly improve with supplemental oxygen.
Another physical examination finding that may be encountered is a holosystolic murmur characteristic of mitral regurgitation, which is often heard in a patient with a primum ASD.
Another physical examination finding that may be encountered is a holosystolic murmur characteristic of mitral regurgitation, which is often heard in a patient with a primum ASD.
IV. LABORATORY EXAMINATION
A. Electrocardiogram (ECG).
The ECG can provide clues to the possibility of an ASD. The rhythm may be sinus, but may also be atrial fibrillation or atrial flutter. Inverted P waves in the inferior leads suggest an absent or nonfunctional sinus node, as may be seen with a sinus venosus defect.
1. Secundum ASD
(a) RSR’ pattern in lead V
(b) QRS duration < 0.11 seconds (incomplete right bundle branch block)
(c) Right-axis deviation
(d) RV hypertrophy
(e) First-degree AV block (20%)
(f) RA enlargement (about 50%) with a prominent P wave in lead II
2. Primum ASD
(a) RSR’ pattern in lead V
(b) Left-axis deviation
(c) First-degree AV block, classically seen with right bundle branch block and left anterior fascicular block
B. Chest radiography
may reveal cardiomegaly due to right heart enlargement. With large left-to-right shunts, the central pulmonary arteries and vascular markings may appear prominent. In the setting of advanced pulmonary vascular disease, however, the pulmonary arteries may appear large but have oligemic peripheral lung fields, so-called vascular pruning.
V. DIAGNOSTIC STUDIES
A. Echocardiography




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree