Fig. 21.1
Clockwise-type typical atrial flutter with 2:1 ventricular response
Conclusions: regular tachycardia, heart rate 150 bpm, incomplete right bundle branch block (100 ms), QRS axis +10°, apparent atrial rhythm at a rate of 300 bpm and 2:1 AV conduction, ST stretch segment from V4 to V6 with flat T waves, and normal corrected QT segment duration
The cardiologist was called to analyze the arrhythmia.
The presence of narrow QRS (100 ms) complex indicates supraventricular tachycardia.
Differential Diagnosis for Narrow QRS Complex Tachycardia
(see Chap. 19)
Sinus tachycardia
Reentrant supraventricular tachycardias
Atrioventricular reciprocating tachycardia
Atrioventricular nodal reciprocating tachycardia
Focal atrial tachycardia
Atrial flutter
Atrial fibrillation
The regular RR interval excludes atrial fibrillation. The presence of an organized atrial rhythm at a rate of 300 bpm, with no isoelectric segment between the typical “sawtooth” waves, best seen in inferior leads, suggests the diagnosis of typical atrial flutter.
The two-to-one conduction makes difficult to recognize the atrial waves superimposed on the QRS complex or T waves, but the cardiologist performed vagal maneuvers (carotid massage) to reveal atrial waves increasing AV block (Fig. 21.2).
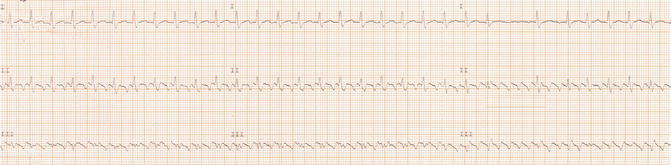

Fig. 21.2
ECG strip of leads I, II, and III shows carotid sinus massage with progressive increase in atrioventricular block
The presence of AV block and the ineffectiveness of carotid massage to terminate the tachycardia exclude the hypothesis of supraventricular reentrant tachycardia. The atrial tachycardia may be excluded because of higher atrial rate and the absence of isoelectric baseline between regular atrial activations.
At the end, the cardiologist concluded a diagnosis of typical atrial flutter.
Routine Laboratory Tests
Complete blood count: normal (Hgb 14.6 g/ dl)
Cholesterol (total, HDL, LDL) and TG: normal
Hepatic function (GOT, GPT, γ-GT, ALP, total bilirubin, direct and indirect): normal
Thyroid function (TSH, FT3, FT4): normal
Renal function (creatinine, BUN): normal
Electrolytes (Na + , K + , Ca ++ , Mg ++ , Cl − ): normal
Fasting blood glucose: 90 mg/dl
HbA1c: 5.0 %
TnI-hs and CK-MB: 0.25 ng/ml (normal <0.055 ng/ml)
BNP: 127 pg/ml (normal 1–100 pg/ml)
The routine laboratory did not show any reversible proarrhythmic trigger such as electrolyte imbalance, anemia, and thyroid dysfunction. The troponin I-hs value was slightly increased due to the tachyarrhythmia. The high myocardial rate may cause an imbalance between myocardial oxygen supply and demand by enhancing myocardial oxygen requirements and reduces the diastolic coronary filling time. Also the BNP value was slightly increased as a result of initial atrial increased filling pressure.
Chest X-Ray
Both lung fields and costophrenic angles are clear. The mediastinum is within normal limits. Cardiac size and shape are normal.
In agreement with patient’s symptoms and physical examination, chest X-ray did not show radiographic signs of pulmonary stasis.
Echocardiography Performed to Assess Cardiac Function and the Presence of Structural Heart Disease
Normal trileaflet aortic valve and normal aorta dimension (aortic root dimension = 2.7 cm; ascending aorta = 3.1 cm; aortic arch = 3.1 cm; thoracic aorta = 2.8 cm; abdominal aorta = 1.8 cm). Mild enlarged left atrium (LA diameter M-mode 41 mm; LA indexed volume 36 ml/m2). Normal right atrium. No atrial septal defect. Mild left ventricular hypertrophy with normal systolic function (mass indexed volume 70 ml/m2; EF 0.65) in the absence of regional wall motion abnormalities. Normal right ventricle size with normal function. Mild mitral and tricuspid regurgitation with normal pulmonary arterial pressure (PAPs = 30 mmHg). No pericardial effusion. Slightly dilated inferior vena cava (24 mm) with >50 % respiratory collapse. Monophasic diastolic flow pattern with initial increase of estimated filling pressure.
Conclusions: hypertensive cardiomyopathy (HCM). The absence of any signs of ventricular dysfunction excludes a tachycardiomyopathy.
The patient was a young active person and he had never had heart rhythm problems before; in fact this episode of arrhythmia was the first in his life. Furthermore it could be triggered by bronchitis.
For these reasons, and in consideration of the presence of only mild left atrium enlargement, the cardiologist chose a rhythm-control strategy. The patient was electrically cardioverted. Catheter ablation is indicated for recurrent, symptomatic, or drug-refractory arrhythmia and therefore was not performed in this case.
The exact duration of atrial flutter was unknown and lasted longer than 48 h. The patient had a CHA2DS2-VASc score of 2, suggesting a considerable risk of stroke.
Many risks and complications of cardioversion are associated with thromboembolic events, so effective anticoagulation for at least 3 weeks is mandatory for AFL of >48 h or AFL of unknown duration, unless the patient was hemodynamically unstable (LGAF).
At this point, the cardiologist proposed to perform a transesophageal echocardiography (TEE) as an alternative to 3 weeks of adequate pre-cardioversion anticoagulation.
In the meantime, a good control of ventricular rate was achieved by administrating a calcium-channel blocker (verapamil).
Transesophageal echocardiography was performed.
Conclusion: TEE excluded the presence of thrombus in the left atrium or left atrial appendage.
The patient was fully informed about the risk of cardioversion procedure.
He was sedated with a light anesthetic (midazolam). Two electrode paddles were placed on his chest: the anterior patch was placed under the right clavicle and the apical patch was placed at the apex (anterior-apical paddle position). Electrical cardioversion was performed during a close oxygen level, blood pressure, and heart rhythm monitoring. Atrial flutter was successfully converted to sinus rhythm after one synchronized biphasic electrical shock at 50 J, without complications (Figs. 21.3 and 21.4).
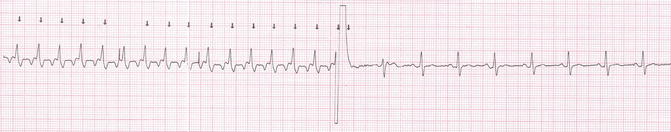
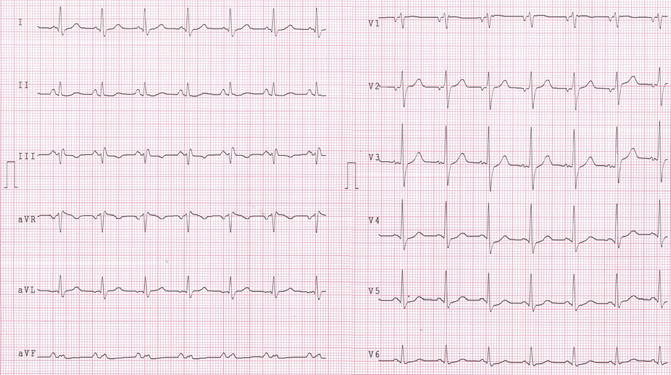

Fig. 21.3
Electrical cardioversion. The atrial flutter is converted to sinus rhythm after one synchronized biphasic electrical shock at 50 J

Fig. 21.4
Sinus rhythm, 85 bpm, normal atrio and inter- ventricular conduction, normal ripolarization
21.2 Atrial Flutter
Atrial flutter (AFL) management includes antithrombotic and antiarrhythmic therapy, correction of possible underlying causes, and treatment of associated comorbidities. The patient had a considerable risk of thromboembolism (CHADS2VASc = 2) and a low risk of bleeding (HAS-BLED = 1). In this case, oral anticoagulant (OAC) therapy is indicated not only for 4 weeks after cardioversion (atrial stunning-related risk), but it should continue lifelong irrespective of an apparent maintenance of sinus rhythm. The cardiologist explained pros and cons of the OAC therapy and following the patient’s preferences prescribed a new oral anticoagulant (NOAC).
Catheter ablation and chronic prophylactic antiarrhythmic therapy were not recommended because it was the patient’s first episode of AFL and his AFL was probably due to a respiratory trigger (bronchitis). It was also well tolerated. Catheter ablation is indicated in case of poorly tolerated or recurrent symptomatic AFL.
Hypertension is a risk factor for atrial flutter and its related complications. The patient referred optimal blood pressure control with systolic blood pressure <140 mmHg and diastolic blood pressure <90 mmHg; the antihypertensive therapy was not changed.
Definition
The atrial flutter is a common supraventricular arrhythmia caused by an intra-atrial macro-reentrant circuit. It is characterized by a rapid and organized atrial depolarization at a rate between 250 and 350 bpm and a different degree of AV nodal blocking [1].
Epidemiology
The Marshfield Epidemiological Study Area (MESA) shows an incidence of atrial flutter of 88/100,000 person-years with 200,000 new cases [2]. Overall, atrial flutter represents approximately 10 % of supraventricular tachycardia, and it is much less common than atrial fibrillation [3]. However atrial flutter occurs in approximately 25–35 % of patients with atrial fibrillation. The incidence is greater in men than in women and increased markedly with age [4].
In 60 % of cases, atrial flutter occurs with a specific precipitating event such as thyroid dysfunction, respiratory infections, pulmonary embolism, or recent major surgery, while in remaining cases, atrial flutter is usually associated with chronic comorbid conditions such as heart failure, valve abnormalities (especially mitral valve), congenital defects, hypertension, chronic lung disease, alcoholism, or use of stimulants such as cocaine, amphetamines, diet pills, and even caffeine. More rarely (only 1.7 % of cases), atrial flutter can occur in the absence of structural heart disease, like idiopathic form (lone atrial flutter) [4]. The possibility of a genetic predisposition is unclear.
Physiopathology
AFLs comprise a heterogeneous group of atrial arrhythmias produced by abnormalities of impulse conduction that are underpinned by macro-reentrant circuits. The mechanism of reentry consists of a repetitive excitation of a specific myocardial region (excitable gap) with consequent conduction of the electrical impulse across a defined circuit, around a fixed obstacle. The macro-reentry circuit is supported by conditions of slowed conduction constrained by barriers that may be anatomical, functional, or both. The development of a unidirectional conduction block starts the arrhythmias, and it is usually a consequence of an acceleration of the heart rate or block of a premature impulse, while for its maintenance, a slow conduction is required [4].
Different types of macro-reentrant are possible.
Atrial flutter and atrial fibrillation may be present in the same person. A common trigger is recognized such as repetitive premature impulse originating from pulmonary veins or from both atria. Furthermore the AF originating from the left atrium may invade the right atrium following macro-reentrant circuit triggering AFL. In addition atrial remodeling consequently to AFL may promote the onset of AF [5].
Atrial Flutter Classification
The classification of atrial flutter proposed by the European Society of Cardiology and the North American Society of Pacing and Electrophysiology is based on both anatomical features and electrophysiological mechanisms, determined at the time of electrophysiological testing [6]. Atrial flutter is categorized into typical or atypical.
Typical Atrial Flutter
Typical atrial flutter is subdivided into two subtypes:
Counterclockwise atrial flutter is the most common type of AFL (90 %). The reentrant circuit is located in the right atrium, whose fundamental component is the isthmus, a posteroseptal part of slow conduction bounded by the inferior vena cava, Eustachian ridge, coronary sinus, and the tricuspid valve annulus. The impulse activation rotates in a counterclockwise direction through the atrial septum, by the superior vena cava, and then inferiorly down the right atrial free wall, through the isthmus to reenter the atrial septum.< div class='tao-gold-member'>Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


