Atrial Fibrillation Trigger Mapping
Fermin C. Garcia
Francis E. Marchlinski
Ablation strategies for atrial fibrillation (AF) have been divided into three general categories for targeting presumed contributors to the pathophysiologic mechanism of AF initiation and maintenance. Ablation or isolation of triggers, elimination of the substrate that potentially allows AF perpetuation, and ablation of the autonomic ganglia that appear to potentiate AF initiation have all been suggested as appropriate strategies. Admittedly, there is continued debate regarding which target is most important for the different forms of AF. In this chapter, we describe methods to provoke and localize AF triggers that we believe play an important role in all forms of AF.
AF is triggered most commonly from foci arising from pulmonary veins (PV) (1). However, non-PV ectopy from different anatomic regions of the right and left atria have also been documented to initiate AF. Our laboratory and others have demonstrated non-PV atrial triggers (Table 15.1) commonly arising from the superior vena cava (SVC), crista terminalis (CT), Eustachian ridge (ER), interatrial septum/fossa ovalis (FO), left atrial posterior wall (LAPW), ligament of Marshall (LOM), tricuspid (TV) or mitral valve (MV) annulus, coronary sinus (CS), and left atrial appendage (LAA) (2, 3, 4, 5). We have divided this chapter into general information about our methods regarding catheter selection, placement and recording techniques for identifying the origin of triggers, and techniques we use for provoking triggers. We then proceed with a more detailed discussion of clues and techniques for identifying and mapping triggers from specific anatomic regions in the atria.
In order to identify AF triggers it is necessary to provoke and record their discharge (6). Antiarrhythmic drug therapy is usually stopped at least five half-lives, or at least 2 weeks, for those patients taking amiodarone. After vascular access has been
obtained, multipolar catheters are positioned in our laboratory in different anatomic regions. A decapolar catheter, capable of internal cardioversion (CV), is positioned in the CS via an internal jugular vein access with the most proximal electrode at the CS ostium (CS os). A second decapolar catheter (crista catheter) is routinely placed in the posterior RA along the CT with the earliest activation in sinus rhythm (SR) recorded in bipole 2 to 3 (distal catheter tip in the SVC). Intracardiac echocardiography (ICE) is rarely necessary to define proper catheter position for these two multipolar catheters. Catheter placement is achieved solely with appropriate attention given to intracardiac recordings and fluoroscopy. These two multipolar catheters, as will be described, are essential for facilitating rapid localization/regionalization of both PV and non-PV triggers. Finally, a His bundle recording may also be deployed when attempting to confirm the diagnosis of AV nodal re-entry or trying to identify a para-Hisian trigger. Some laboratories also use a His catheter recording to more rapidly identify right atrial triggers (7).
obtained, multipolar catheters are positioned in our laboratory in different anatomic regions. A decapolar catheter, capable of internal cardioversion (CV), is positioned in the CS via an internal jugular vein access with the most proximal electrode at the CS ostium (CS os). A second decapolar catheter (crista catheter) is routinely placed in the posterior RA along the CT with the earliest activation in sinus rhythm (SR) recorded in bipole 2 to 3 (distal catheter tip in the SVC). Intracardiac echocardiography (ICE) is rarely necessary to define proper catheter position for these two multipolar catheters. Catheter placement is achieved solely with appropriate attention given to intracardiac recordings and fluoroscopy. These two multipolar catheters, as will be described, are essential for facilitating rapid localization/regionalization of both PV and non-PV triggers. Finally, a His bundle recording may also be deployed when attempting to confirm the diagnosis of AV nodal re-entry or trying to identify a para-Hisian trigger. Some laboratories also use a His catheter recording to more rapidly identify right atrial triggers (7).
TABLE 15.1 Incidence and Location of Non-Pulmonary Vein Triggers | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||
Programmed electrical stimulation (PES) in the atria (from CS or crista catheter) and ventricle is performed to exclude the presence of atrioventricular nodal reentrant tachycardia (AVNRT) or an accessory AV pathway. A detailed stimulation protocol is typically not necessary, but an assessment of VA conduction and atrial stimulation sufficient to stress AV nodal conduction and bring out dual AV nodal pathway physiology should be routine if SR is present. If dual-pathway physiology with at least a single echo beat is observed, programmed stimulation on low-dose isoproterenol is performed to determine the inducibility of sustained AVNRT/AVRT. These supraventricular tachycardias (SVTs) are considered a non-PV trigger if they degenerate into AF at any time after induction or if they are the only arrhythmia initiated and no PV or other non-PV triggers for AF can be identified with higher-dose isoproterenol-provocative maneuvers (Fig. 15.1 and 15.2). We have found this effort of particular importance in young patients with a longstanding history of palpitations.
Identification of the SVT and successful ablation has eliminated the need for transseptal puncture and provided long-term elimination of AF in selected patients (8).
Identification of the SVT and successful ablation has eliminated the need for transseptal puncture and provided long-term elimination of AF in selected patients (8).
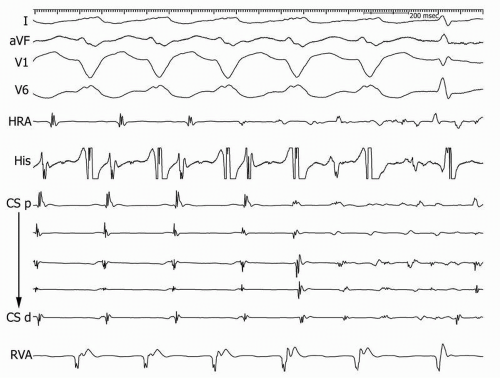
 Figure 15.1. Stable typical AVNRT with CL 280 ms is present for the first four beats of the figure and then degenerates into AF. CS, coronary sinus; p, proximal; d, distal; RVA, right ventricle apex. |
Importantly, we have identified AVNRT even in patients with persistent or permanent forms of AF, so we insist on doing stimulation to exclude AVRNT or AVRT at the end of the ablation procedure in all patients when they present with more
sustained forms of AF after that arrhythmia has been eliminated. We found AVNRT present in 27 of 629 (4.3%) consecutive patients undergoing AF catheter ablation. If no SVT is provoked or if the patient is in a more persistent form of AF at the beginning of the procedure and the clinical history or ECG recording suggests a low probability of SVT triggering AF, we routinely perform double transseptal punctures under ICE guidance to insert a circular mapping catheter of appropriate size for the PVs (based on ICE assessment) and a quadripolar mapping/ablation catheter. The catheters are advanced into the left atrium (LA) via either a Mullins transseptal sheath or curved/steerable sheaths and positioned in the right and left PVs prior to provocative maneuvers to identify PV or non-PV triggers. Our catheter placement as shown (Fig. 15.3) allows for systematic analysis of activation patterns associated with
spontaneous or provoked triggers and rapid regionalization of the origin of the trigger. More-detailed mapping can then be performed to confirm the precise site for ablation/isolation.
sustained forms of AF after that arrhythmia has been eliminated. We found AVNRT present in 27 of 629 (4.3%) consecutive patients undergoing AF catheter ablation. If no SVT is provoked or if the patient is in a more persistent form of AF at the beginning of the procedure and the clinical history or ECG recording suggests a low probability of SVT triggering AF, we routinely perform double transseptal punctures under ICE guidance to insert a circular mapping catheter of appropriate size for the PVs (based on ICE assessment) and a quadripolar mapping/ablation catheter. The catheters are advanced into the left atrium (LA) via either a Mullins transseptal sheath or curved/steerable sheaths and positioned in the right and left PVs prior to provocative maneuvers to identify PV or non-PV triggers. Our catheter placement as shown (Fig. 15.3) allows for systematic analysis of activation patterns associated with
spontaneous or provoked triggers and rapid regionalization of the origin of the trigger. More-detailed mapping can then be performed to confirm the precise site for ablation/isolation.
The protocol used in our laboratory in an attempt to provoke and identify AF triggers is systematically performed and has allowed us to identify the most common sites of origin of triggers and determine the overall incidence of provoked triggers.
The initial step to provoke AF will depend on the presence of AF at the time of catheter placement. If the patient is in AF, CV is performed to identify spontaneous occurrence of triggers reinitiating AF, also known as early recurrence of atrial fibrillation (ERAF). If the patient is in SR or SR is restored with CV and no spontaneous triggers are evident, isoproterenol is administered as a continuous infusion. The initial dose is 3 μg/min for 5 minutes, with escalating doses of 6, 12, and 20 μg/min incremented at 3- to 5-minute intervals. Most patients tolerate high-dose isoproterenol with the median dose achieved of 12 μg/min required for AF trigger identification. In our experience, over 70% of patients will develop atrial premature depolarizations (APDs) and triggers for AF in response to this incremental-dose isoproterenol administration.
If no AF triggers are identified, burst pacing is performed to initiate AF and CV is performed to look for ERAF. If triggers are not identified following CV of induced or spontaneous AF, then the induction of AF is repeated and CV is performed during low-dose isoproterenol infusion (2 μcg/min). Using these provocative maneuvers, we have identified reproducible AF triggers, defined as recurrent APDs or APDs triggering AF, form the PVs or non-PV triggers initiating AF in over 90% of patients who are undergoing AF ablation procedures.
The PVs are clearly the most common origin of AF triggers (Fig. 15.4). Of those patients with spontaneous or provocable triggers, approximately 95% will demonstrate PV triggers alone or in combination with non-PV foci. It is because of this high frequency of PV triggers that many centers have abandoned provocative maneuvers before PV isolation. We continue to attempt to identify origin of triggers before PV isolation for three reasons. First, the ability to limit ablation remains important and we believe that the identification of patients with only non-PV triggers permits a limited ablation with good outcome. Second, we have found that young patients with paroxysmal atrial fibrillation (PAF) may benefit from selective targeting of only one or two PVs that demonstrate triggers (9). This approach is reserved for patients without structural heart disease with normal LA size that also do not have other AF risk factors such as hypertension, obesity, or sleep apnea. Finally, we believe that knowledge of a specific PV trigger site allows one to target that PV or ipsilateral PVs for isolation initially and allows for the maximum period of observation to identify early PV reconnection.
Right versus left PV ectopy can be differentiated by analyzing the activation time between the CS catheter and the CT catheter during the ectopy. By determining the time interval between the RA recordings from the CT catheter and the CS catheter coupled with an analysis of the activation pattern noted in the multipolar CS catheter
recordings, one can determine right versus left PV origin for the trigger. Right PV ectopy activates the RA rapidly via Bachmann’s bundle and activates the CS recordings with a proximal-to-distal activation sequence. The activation in the RA CT catheter recording will always precede the CS os recording by at least 15 to 20 ms. In contrast, the left PVs typically activate the CS catheter before the RA CT catheter and the CS activation sequence is typically distal to proximal. Occasionally, the CS activation will show the mid-set of electrodes activated earliest and RA CT and CS activation near simultaneously. This will occur with a left superior PV trigger and a high takeoff for the left superior PV near the LA roof. This pattern of CS activation can also be seen associated with triggers from a posterior and laterally displaced right inferior PV. Similar findings to differentiate right from left PV ectopy have been confirmed by other investigators (4,7,10,11).
recordings, one can determine right versus left PV origin for the trigger. Right PV ectopy activates the RA rapidly via Bachmann’s bundle and activates the CS recordings with a proximal-to-distal activation sequence. The activation in the RA CT catheter recording will always precede the CS os recording by at least 15 to 20 ms. In contrast, the left PVs typically activate the CS catheter before the RA CT catheter and the CS activation sequence is typically distal to proximal. Occasionally, the CS activation will show the mid-set of electrodes activated earliest and RA CT and CS activation near simultaneously. This will occur with a left superior PV trigger and a high takeoff for the left superior PV near the LA roof. This pattern of CS activation can also be seen associated with triggers from a posterior and laterally displaced right inferior PV. Similar findings to differentiate right from left PV ectopy have been confirmed by other investigators (4,7,10,11).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree