Atrial Fibrillation in the Emergency Department
Michael A. Ross
Antonio X. Bonfiglio
Epidemiology
The most common clinically important disturbance in cardiac rhythm seen in the emergency department (ED) is atrial fibrillation (AF) (1). An estimated 2.2 million Americans have paroxysmal or persistent AF. AF affects approximately 2% of the U.S. population in the sixth decade of life, and its prevalence increases with advancing age. In patients over 75 years old, greater than 10% have experienced AF. AF is also associated with other conditions corresponding with advanced age such as coronary artery disease, hypertension, congestive heart failure, valvular heart disease, hyperthyroidism, and diabetes (2,3).
Because of its higher prevalence in the elderly and the growth of that age group, AF has become a rising cause of hospitalization. AF accounts for 34.5% of admissions for a cardiac rhythm disturbance. Complications of AF range from cardiac decompensation to thromboembolic events such as an acute ischemic stroke. The relative risk of death in patients with AF is one and a half times greater in men and almost two times greater in women (3,4).
Classification
Several terms used in reference to AF must first be understood. Acute onset AF is defined as having been present for less than 48 hours. This distinction is important because it will direct therapy in selected cases. Paroxysmal, or recurrent, AF is that which has occurred at least twice and terminated. Persistent AF is that which does not terminate. Subsequent termination of AF does not change the designation as persistent. If AF persists for more than 1 year, then it is classified as long standing. Lone AF is defined as that occurring in a patient who is under 60 years of age and without echocardiographic or clinical evidence of cardiovascular disease. It is estimated that lone AF represents between 12% and 30% of all AF patients. These classifications do not apply to AFs that last less than 30 seconds or are secondary to precipitating conditions such as cardiac surgery, myocarditis, acute myocardial infarction (MI), hyperthyroidism, or acute pulmonary disease (4).
Pathophysiology
Histological analysis of atrial tissue in patients in chronic AF shows changes beyond those expected by the underlying disease process itself. There is often a mixture of patchy fibrosis or inflammation and normal atrial tissue accounting for nonhomogeneity of atrial refractoriness. Histological changes of myocarditis have been found in atrial biopsy specimens of 66% of patients with lone AF. Atrial fiber hypertrophy and atrial dilation can be demonstrated by biopsy or echocardiography in patients with long-standing AF (4). Selected toxic and metabolic conditions may also contribute to AF.
The causes of AF are many and can be both intrinsic to the heart itself or extrinsic, secondary to other conditions. Two useful pneumonics regarding causes of AF divide causes into noncardiac causes and cardiac causes. Noncardiac causes can be summarized by the pneumonic TRAPS and include Thyrotoxicosis, Recreational drug use (sympathomimetics and endogenous catecholamines), Alcohol, Pulmonary disease (including pulmonary embolus), and Sepsis, or infection. Cardiac causes can be summarized by the pneumonic CATCH WAVE and include Congestive heart failure, Acute coronary syndromes, TaChy-brady syndrome (sick sinus syndrome), Hypertension, Wolff-Parkinson-White syndrome, After cardiac surgery, Valvular heart disease, and mEdical noncompliance (Table 8-1) (5,6,7,8).
In terms of atrial electrical activity, AF occurs through two processes—enhanced depolarization of one or several foci and re-entry involving one or several circuits. Usually AF starts when a trigger, often a premature atrial contraction or repetitive depolarization, finds its way into an aberrant circuit where it does not encounter refractory myocardium (4,9). Electrophysiological mapping studies have found that AF often originates in the muscular sleeve of the pulmonary veins and then propagates through the atrial tissue. A re-entrant impulse is one that travels repetitively along an abnormal electrical circuit. If the propagation of the atrial depolarization is along one re-entrant pathway, this can result in regular arrhythmic depolarizations and atrial flutter. However, more chaotic and irregular electrical activity along multiple circuits is usually the case and leads to AF. These depolarizations compete for the atrioventricular
(AV) node and, depending on their magnitude and time of arrival, may be propagated through the AV node and into the ventricle causing a ventricular contraction (4,10). Often, in a healthy nodal conduction system, the ventricular response is fast producing an irregular tachycardia. On a cellular level, AF has been found to essentially propagate itself. Actual atrial electrical remodeling occurs with continued AF. This involves the down regulation of calcium channels, shortening action potential duration, thus decreasing the refractory period of the myocardial tissue (4,9). A regular ventricular response in light of the chaos in atrial conduction can also be indicative of AV nodal dissociation.
(AV) node and, depending on their magnitude and time of arrival, may be propagated through the AV node and into the ventricle causing a ventricular contraction (4,10). Often, in a healthy nodal conduction system, the ventricular response is fast producing an irregular tachycardia. On a cellular level, AF has been found to essentially propagate itself. Actual atrial electrical remodeling occurs with continued AF. This involves the down regulation of calcium channels, shortening action potential duration, thus decreasing the refractory period of the myocardial tissue (4,9). A regular ventricular response in light of the chaos in atrial conduction can also be indicative of AV nodal dissociation.
Table 8-1. Causes of Atrial Fibrillation | ||||
|---|---|---|---|---|
|
On the electrocardiogram (ECG) AF is characterized by a lack of defined and regular atrial depolarization resulting in an absence of discernible P-waves and chaotic and irregular undulations in their place, often called f-waves. These f-waves occur at rates often between 400 and 600 beats per minute. With atrial flutter, the rates range from 250 to 350 beats per minute. Induction through the AV node is variable, often leading to an irregular depolarization and contraction by the ventricle. The gross appearance on the ECG is the absence of P-waves with varying ventricular contractions that are often irregular. This can be seen with or without other conduction disturbances like bundle branch blocks or accessory pathways and is independent of the morphology of the tracing (6,11). In atrial flutter the typical rate of the atrial flutter waves is roughly 240 to 320 beats per minute. When patients in atrial flutter experience AV nodal conduction rates of 2:1, then the resulting rhythm is a regular tachycardia of roughly 150 beats per minute. Often the nonconducted flutter wave is buried in the preceding T-wave, giving the appearance of a sinus tachycardia with a rate of 150. Atrial flutter with 2:1 conduction should be suspected in any patient with a tachycardia of 150 beats per minute, especially if it is a narrow complex (11).
Once the heart’s intrinsic rhythm is altered by AF, then its function and blood flow are also altered. Cardiac output and stroke volume decline from a lack of adequate preload filling of the ventricle by the contributions of the atrial contraction (12). This results in the loss of the “atrial kick” that would otherwise be provided with normal filling times as well as the loss of organized and rhythmic atrial activity. The irregularity of the ventricular response as well as the tachycardia of the ventricle can also lead to decreased cardiac output. Poor cardiac output in the presence of underlying coronary artery disease or cardiomyopathy will precipitate to congestive heart failure (CHF) or exacerbate acute cardiac ischemia. On the other hand, CHF can cause AF, making this a rhythm disturbance that can become self-propagating in congestive heart failure patients. The turbulence of blood in the atria that occurs in AF can cause blood to pool and clot, particularly in the left atrial appendage. Because of this, patients are at a substantial thromboembolic risk and require anticoagulation based on their risk profile (see Table 8-6) (12,13).
Management of Atrial Fibrillation
There are four primary issues in the management of AF: (a) ventricular rate control, (b) restoration of sinus rhythm, (c) anticoagulation issues, and (d) treatable causes of AF. Underlying treatable causes were described earlier and will not be discussed in this chapter. See Figure 8-1 for a general outline of the treatment of AF (12).
Initial Evaluation
Several issues need to be addressed in the initial evaluation of patients with AF. These include the hemodynamic stability of the patient, whether the symptoms have been present for more or less than 48 hours, and what may have caused the AF to occur. AF of more than 48 hours duration is more likely to form an intracardiac thrombus, which may subsequently embolize if conversion to sinus rhythm occurs. As such, it is important to determine if the onset of symptoms is less than 48 hours (14,15,16). If the symptom onset cannot be clearly determined, then it may be prudent to consider the onset to be greater than 48 hours and focus on rate control rather conversion to sinus rhythm. It is also important to characterize whether the symptoms are an isolated event or a paroxysmal recurrent event because this may affect long-term treatment decisions. The history should also focus on identifying potential causes that may have triggered AF and to focus appropriate testing (14).
The clinical presentation of AF in the ED ranges from the asymptomatic patient in whom it is an incidental finding, to those with an uncomplicated acute onset of symptoms, and to those suffering an acute end organ compromise by hypoperfusion or thromboembolization (17). Symptomatically, AF may or may not produce symptoms that allow the patient to identify the exact time of onset. Patients may describe the onset as a sudden feeling of palpitation, weakness, dizziness, syncope, or dyspnea. The examination of patients in AF will usually suggest the arrhythmia by the classical irregularly irregular pulse, irregular venous pulsations, and variation in the loudness of the first heart sound (4,12,14). The patient’s blood pressure is critically important in the identification of patients that are in hypotensive shock and may require urgent cardioversion (12,18). The temperature can point to sepsis, or thyrotoxicosis, as an etiology. Physical examination findings can also point to etiologies such as CHF, pulmonary disease, thyroid disease, or recent cardiac surgery.
All patients with palpitations or symptoms suggestive of AF should have an initial ECG performed to confirm the rhythm, as well as identify confounding issues such as evidence of an acute coronary syndrome, Wolff-Parkinson-White (WPW) syndrome, or electrolyte disturbances such as hypokalemia (4). These confounders may have an impact on selecting therapies. Additionally, patients should have an intravenous line placed,
receive supplemental oxygen if they are hypoxic or symptomatic, and be placed on a cardiac monitor to follow their heart rate. This will help determine if and when a patient returns to a normal sinus rhythm. Cardiac monitors may not provide an accurate measure of the patient’s heart rate because most cardiac monitoring equipment determines the heart rate by measuring the R to R interval, and this is irregular in AF (7). When in doubt, it is most accurate to count the number of complexes on a rhythm strip over a fixed time interval, such as 6 seconds, which is then multiplied by ten, to determine the patient’s heart rate. Following conversion to sinus rhythm patients should have an ECG repeated and remain on the monitor for at least a brief period of time because some will continue to go in and out of AF (7,10).
receive supplemental oxygen if they are hypoxic or symptomatic, and be placed on a cardiac monitor to follow their heart rate. This will help determine if and when a patient returns to a normal sinus rhythm. Cardiac monitors may not provide an accurate measure of the patient’s heart rate because most cardiac monitoring equipment determines the heart rate by measuring the R to R interval, and this is irregular in AF (7). When in doubt, it is most accurate to count the number of complexes on a rhythm strip over a fixed time interval, such as 6 seconds, which is then multiplied by ten, to determine the patient’s heart rate. Following conversion to sinus rhythm patients should have an ECG repeated and remain on the monitor for at least a brief period of time because some will continue to go in and out of AF (7,10).
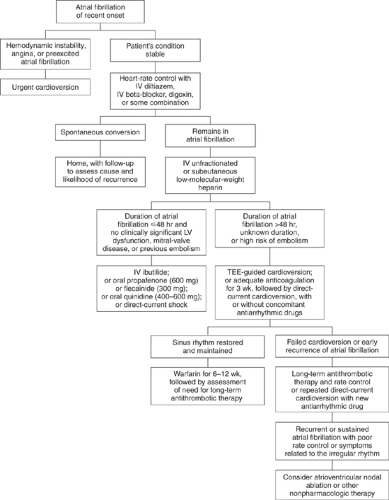 Figure 8-1. Management of recent onset atrial fibrillation. (From Falk RH. Atrial fibrillation. N Engl J Med 2001;344:1067–1078, with permission.) |
Given the multiple causes of AF, selected testing can be performed to determine the cause. Specifically, if an acute coronary syndrome is suspected, then cardiac markers such as creatine kinase-MB (CK-MB) or cardiac troponins may be ordered. Pulse oximetry should be considered to help identify patients that are hypoxic from CHF or pulmonary embolism (PE). Brain natriuretic peptide (BNP) may be used as a screening tool to identify patients with CHF as a cause, or result, of AF. Electrolytes and renal function labs may be of value in these patients. Coagulation studies and a complete blood count with platelets may be indicated for patients taking warfarin or heparin, or for patients at risk of bleeding such as those with liver or bone marrow failure. Thyroid function testing or toxicology screening can be ordered as needed as well. A chest x-ray may be useful to identify CHF or pneumonia in the dyspneic patient. If clinically indicated, then imaging for a pulmonary embolus may be needed. An ECG may help identify if a patient is in CHF (4,7,14,18,19). Transesophageal echocardiography may be useful in identifying a clot in the left atrial appendage; however, this study is usually performed in settings other than the ED (15,16).
Treatment of Atrial Fibrillation
Emergent Electrical Cardioversion of Unstable Atrial Fibrillation Patients
For patients with unstable AF, cardioversion is the treatment of choice. This would include patients that are hypotensive or with other signs of shock, such as acute mental status changes due to cerebral hypoperfusion, and ongoing severe ischemic chest pain. These symptoms often only occur when the heart rate exceeds 150 beats per minute. Patients with acute MI and AF with a rapid ventricular response should be electrically cardioverted if they do not promptly respond to medical therapy. Cardioversion is also often the preferred method for AF patients with pre-excitation syndromes such as WPW (7,12,18).
The electrical cardioversion of AF or flutter should be done in the synchronized mode to avoid shock during the relative refractory period, which can lead to ventricular fibrillation. If synchronized shocks are not possible because of the patient’s rhythm or condition, then a higher energy shock should be used (defibrillation doses). When cardioverting AF in a monophasic mode, a setting of 100 to 200 joules (J) is often needed. However, when using a biphasic synchronized cardioverting waveform, a setting of 100 to 120 J is usually adequate to convert the patient. When cardioverting atrial flutter, a lower monophasic setting of 50 to 100 J is usually sufficient (18).
Medical Management of Atrial Fibrillation—Medications to Control Heart Rate
The heart rate in AF with a rapid ventricular response may be controlled using a number of medications that include beta-blockers, calcium channel blockers, and magnesium. These drugs work by blocking conduction at the AV node. Doses and major side effects are listed in Table 8-2. Because of its very short duration of action, adenosine should not be used to manage AF or flutter. Because of its late onset of action and marginal efficacy, digoxin is only listed as a class IIb medication in the setting of CHF. In the ED, rate control should be achieved if the patients’ ventricular response is greater than 120 beats per minute. In outpatients, the heart rate is considered controlled when it is between 60 to 80 at rest and 90 to 115 during moderate exercise. Therapy requires careful titration and bradycardia may occur, though often only transiently. In general, for patients with good left ventricular (LV) function, beta-blockers or calcium channel blockers may be used. For patients with CHF, diltiazem, amiodarone, and digoxin may be used. For patients with pre-excitation conditions (i.e., WPW) and AF, rate control may be achieved with amiodarone, propafenone, procainamide, flecainide, or sotalol. In pre-excitation conditions, beta-blockers, calcium channel blockers, digoxin, and adenosine are contraindicated. For patients with chronic AF, control of a rapid ventricular response is a common clinical presentation. Medications to control the ventricular rate are often the mainstay of their treatment in the ED (4,7,12,18,19).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


