Fig. 3.1
Gesell’s description of the association between atrial fibrillation and a drop in the arterial pressure
Atrial fibrillation (AF) affects more than 2 million patients in the United Sates, and the prevalence will continue to increase as the population ages [2]. Atrial fibrillation and heart failure have an intimate and bidirectional relationship: atrial fibrillation exacerbates heart failure, and heart failure increases the risk of atrial fibrillation. Many of the risk factors for atrial fibrillation such as diabetes, hypertension, and coronary artery disease are common risk factors for heart failure [2]. In a study by Wang et al., 1470 patients were followed for 5.6 years after atrial fibrillation diagnosis, and 4.2 years after heart failure diagnosis, finding that 42 % of patients with AF developed or had congestive heart failure (CHF), and 41 % of CHF patients developed AF. In addition, the prevalence of AF increases with advancing New York Heart Association (NYHA) functional class, from <10 % in NYHA Class I to 50 % in those with NYHA functional Class IV [3, 4].
Numerous clinical trials have demonstrated that AF increases mortality in patients with heart failure. For instance, in the SOLVD trial, patients with AF had higher mortality than patients with sinus rhythm at baseline (34 % vs. 23 %; p <0.001), even after adjusting for clinical parameters such as left ventricular ejection fraction (LVEF), NYHA functional class, and age [5].
Beneath the surface of the epidemiological relationship, translational experiments have elucidated the direct relationship between elevated atrial pressure and the threshold for AF initiation. Increased atrial pressure not only reduces the atrial effective refractory period but also increases the dispersion of atrial refractoriness [6]. These two factors work in concert to promote a substrate for AF inducibility and sustainability.
Atrial fibrillation leads to several physiological consequences: loss of atrial systole, irregular ventricular rhythm, increased ventricular rate, and loss of physiological control of the heart rate. These physiological changes during atrial fibrillation underlie the clinical effects, such as reduced cardiac output, exacerbation of diastolic and systolic heart failure, imbalance between myocardial oxygen supply and demand as a result of impaired coronary perfusion and reduced exercise capacity [2].
Pardeans et al. demonstrated that AF is associated with a 20 % lower peak VO2 in heart failure patients, highlighting the importance of maintaining cardiac output and exercise performance in patients with impaired ventricular function [7].
Naturally, the relationship between AF and CHF is far more complex than simply pressure overload-induced electrical changes. A schematic representation of the complex and multifaceted relationship between the pathophysiology of AF and CHF was elegantly described by Maisel et al. [2]. Heart failure leads to volume and pressure overload, irregular ventricular filling, neurohormonal activation, atrial enlargement, atrial interstitial fibrosis, calcium dysregulation, and altered atrial electrical properties, all of which promote atrial fibrillation. In terms of fibrosis, it has been noted on histological studies that it is mostly distributed in the posterior wall, has a direct correlation with fractionated potentials found during mapping and ablation while the dominant frequency is lower with a higher organizational index [3]. The location of fibrosis has a major impact in techniques of AF ablation in CHF patients as illustrated later. Atrial fibrillation leads to cellular and extracellular remodeling, loss of AV synchrony, rapid ventricular response, and lower cardiac output, promoting heart failure [2, 8] (Fig. 3.2).
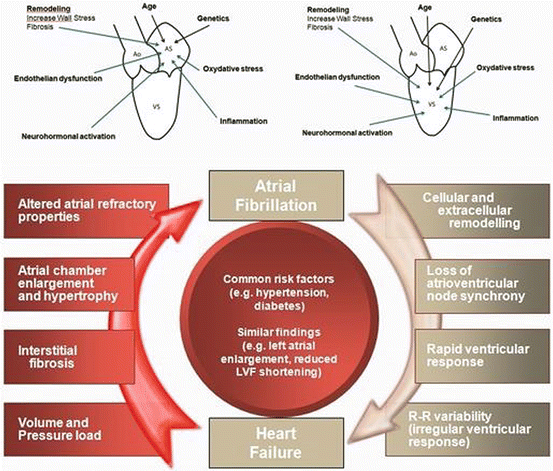

Fig. 3.2
There are multiple facets underlying the common pathophysiology of heart failure and atrial fibrillation. Heart failure leads to volume and pressure overload in the atrium and ventricle. This leads to neurohormonal activation, promoting interstitial fibrosis, atrial chamber enlargement, and endothelial dysfunction, which can alter atrial refractory properties and lead to atrial fibrillation. Atrial fibrillation leads to loss of atrioventricular synchrony, rapid ventricular response, and variable R-R intervals, which thereby promote heart failure. This can lead to a vicious cycle in which heart failure begets atrial fibrillation, and atrial fibrillation begets heart failure (center panel) (Reproduced with permission from [2])
One of the important links between AF and CHF is the upregulation of the neurohormonal system. Neurohormonal activation is a well-established consequence of heart failure and represents the most important target of pharmacotherapy. Angiotensin II, a critical octapeptide hormone of the renin-angiotensin-aldosterone cascade, can cause increased extracellular matrix fibrosis, which can alter atrial conduction properties and refractory periods, predisposing to the development of atrial fibrillation [9].
3.1 Treatment of Atrial Fibrillation in the Heart Failure Population
Having the pathophysiologic changes in mind, it is obvious that optimal medical therapy for heart failure may also help improve atrial fibrillation, including the use of medications targeting the renin-angiotensin-aldosterone pathway. For example, angiotensin-converting enzyme inhibitors (ACE-I), a mainstay of afterload reduction in heart failure, have been shown to reduce atrial fibrillation risk in heart failure. Clinical studies and pathological correlation demonstrate that ACE inhibition may help decrease left atrial fibrosis and the risk of AF recurrence after cardioversion [10]. In addition, angiotensin receptor blockade has also been shown to reduce the incidence of atrial fibrillation in patients with heart failure [11].
Beta-blockers, another mainstay of pharmacotherapy for heart failure, improve atrial fibrillation as well. In a meta-analysis of seven studies including 11,952 patients receiving angiotensin-converting enzyme inhibitors, treatment with beta-blockers was associated with 27 % relative risk reduction in the incidence of AF (RR 0.73, 95 % CI:0.61–0.86, p = 0.001) [12].
Given the increased morbidity and mortality associated with growing epidemic of both heart failure and atrial fibrillation, it is of particular scientific and clinical importance to find optimal treatment strategies. For atrial fibrillation treatment, these can be divided into two main categories: rate control or rhythm control.
Rate control entails medical therapy for suppression of rapid ventricular response, or can involve, in some cases, atrioventricular node ablation and pacemaker implantation.
Rhythm control includes antiarrhythmic drugs and ablation of atrial fibrillation. The optimal strategy has been the subject of numerous clinical trials in the atrial fibrillation population at large, as well as in the subgroup of patients with heart failure.
The presence of systolic heart failure renders the treatment pharmacological strategies with rate control or rhythm control more complex. Systolic heart failure, for example, precludes the use of certain antiarrhythmic medication, such as Class Ic drugs (flecainide and propafenone), that may have a negative inotropic effect. In addition, rate control medications may be limited by poor tolerance and hypotension. Several clinical trials in the atrial fibrillation population at large have failed to show benefit of rhythm control over rate control. For instance, the AFFIRM trial compared rate control strategy with a rhythm control strategy. In the trial, 4060 patients were enrolled and randomized to either rate control (digoxin, beta-blocker, diltiazem, verapamil) or rhythm control (most commonly amiodarone and sotalol). At 5-year follow-up, the mortality rate in the rhythm control arm vs. the rate control arm was 23.8 % vs. 21.3 %, respectively, HR 1.15 (95 % CI 0.99–1.34; P = 0.08) [13].
Therefore, a rhythm control strategy showed no benefit over a rhythm control strategy, and there was a nonsignificant trend toward increased mortality in the rhythm control arm. Of note, the increased mortality in the antiarrhythmic group was due to increased frequency of malignancies, unlikely related to medications, but rather a possible coincidence.
As a result of the AFFIRM trial, it was thought that the clinical benefit of restoring sinus rhythm is typically offset by the negative side effects of antiarrhythmic drugs. It is important to recognize that the AFFIRM trial was performed in the early era of ablation, and therefore, a very small proportion of patients received catheter or surgical ablation for atrial arrhythmia (total of 18 patients). Finally, a minority of the patients in AFFIRM had depressed left ventricular function and/or advanced NYHA Class, making it difficult to extrapolate these findings to patients with systolic heart failure.
Additional studies aimed to answer this question and included the Pharmacological Intervention in Atrial Fibrillation (PIAF), How to Treat Chronic Atrial Fibrillation (HOT CAFE), and Strategies of Treatment of Atrial Fibrillation (STAF). These trials had similar findings that rhythm and rate control strategies were equivalent, although a high proportion of patients in the rhythm control arms did not maintain sinus rhythm long term [8].
Because the rhythm control arm of the AFFIRM trial had a low proportion of patients maintaining sinus rhythm, a subsequent study analyzed the subgroup of patients who maintained sinus rhythm, using an on-treatment analysis. Interestingly, covariates that were associated with improved survival include maintenance of sinus rhythm (HR 0.54, 95 % CI 0.42–0.70, P <0.0001) and warfarin use (HR 0.47, 95 % CI 0.36–0.61, P <0.0001) [14].
However, it is possible that sinus rhythm was simply a confounder for a healthier patient population. Consistent with prior studies, antiarrhythmic drug use was associated with increased mortality [14]. It is also important to recognize that patients with highly symptomatic atrial fibrillation would not likely be randomized to such clinical trials or alternatively would have typically crossover from the rate control to the rhythm control arms. The Atrial Fibrillation and Congestive Heart failure (AF-CHF) trial sought to examine the rhythm vs. rate control question in the heart failure population. In this multicenter, randomized trial of patients with left ventricular ejection fraction of 35 % or less and AF, there was no difference in cardiovascular mortality between the rhythm control group and the rate control group (27 % vs. 25 %, respectively (HR 1.06; 95 % CI 0.86–1.30; P = 0.59) [15].
In addition, there was no significant difference between the groups with regard to death from any cause, stroke, or worsening heart failure. The vast majority of the patients in the rhythm control arm were taking amiodarone, and maintenance of sinus rhythm was roughly 80 % in the rhythm control arm. The proportion of patients in the rhythm control group requiring hospitalization was higher than the rate control group, which was statistically significant in the first year, likely due to the need for repeat cardioversion or medication adjustments (46 % vs. 39 %, P = 0.001). However, patients did not undergo catheter ablation of atrial fibrillation as part of the rhythm control strategy. Therefore, the AF-CHF study extends the findings of AFFIRM to the systolic heart failure population, showing no benefit of a rhythm control strategy over a rate control strategy [8, 14].
A relatively newer Class III agent, dofetilide, was approved by the FDA in 1999 and today is one of the cornerstones of antiarrhythmic drug therapy in patients with systolic heart failure. This medication requires inpatient loading of the medication for close QT interval and arrhythmia monitoring. In the DIAMOND congestive failure substudy, dofetilide was more effective than placebo in maintaining sinus rhythm in patients with AF and heart failure (79 % with dofetilide versus 42 % with placebo P = 0.001) and also reduced the hospitalization rate for heart failure [16].
While prior trials of AAD and heart failure show a signal for increased mortality or heart failure, the DIAMOND substudy showed no effect on all-cause mortality; restoration and maintenance of sinus rhythm was associated with a reduction of mortality (RR 0.44, 95 % CI 0.30–0.64; P <0.0001), consistent with the AFFIRM substudy described above. The risk for torsade de pointes (TDP) among patients treated with dofetilide was relatively small, 2.1 %. Independent predictors of the development of Tdp were female gender, NYHA Class III/IV, and higher QTc [17].
Finally, in an observational study assessing the effects of CRT and AV node ablation vs. CRT and rate control in patients with atrial fibrillation, CRT and AV node ablation was associated with a ninefold lower heart failure mortality compared to patients with AF who were received CRT and rate control medications only. This was thought to be due to “complete” heart rate control and hence maximal CRT benefit [18].
Based on our current knowledge, it appears that the benefits of sinus rhythm may be neutralized by the negative effects of antiarrhythmic medication is a recurrent theme of debate in the rate vs. rhythm control. In addition, antiarrhythmic drugs do not have high efficacy in the long term, the options of drugs are limited, and the side effects can be considerable.
Catheter ablation for AF has rapidly been recognized as a highly effective treatment option for AF that is refractory to pharmacological therapy. Many patients undergoing successful ablation may cease their antiarrhythmic medication, avoiding then the potential negative side effects encountered with long-term use. Catheter-based AF ablation is focused not only on pulmonary vein isolation but also associated additional linear ablation or complex fractionated electrogram ablation in patients with CHF, as these patients typically have a different mechanism of atrial fibrillation. While in paroxysmal atrial fibrillation it has been recognized that pulmonary vein foci are the main triggers, in persistent AF usually associated with CHF, there are additional substrates for AF – posterior wall fibrosis, foci outside the pulmonary veins, and extensive scarring in the left atrium. A newer technology, cryoballoon ablation for pulmonary vein isolation, has also recently been approved and is also widely being used clinically; however, its utility in persistent AF and CHF remains to be determined.
In radiofrequency catheter ablation, various lesion sets and techniques have been applied [19] (Fig. 3.3).
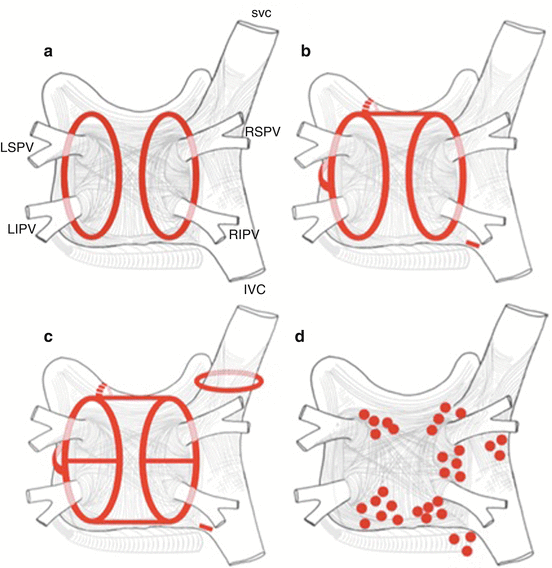

Fig. 3.3
Schematic of common lesion sets employed in AF ablation. A: The circumferential ablation lesions that are created in a circumferential fashion around the right and the left PVs. The primary endpoint of this ablation strategy is the electrical isolation of the PV musculature. B: Some of the most common sites of linear ablation lesions. These include a “roof line” connecting the lesions encircling the left and/or right PVs, a “mitral isthmus” line connecting the mitral valve and the lesion encircling the left PVs at the level of the left inferior PV, and an anterior linear lesion connecting either the “roof line” or the left or right circumferential lesion to the mitral annulus anteriorly. A linear lesion created at the cavotricuspid isthmus is also shown. This lesion is generally placed in patients who have experienced cavotricuspid isthmus-dependent atrial flutter clinically or have it induced during EP testing. C: Similar to 3B but also shows additional linear ablation lesions between the superior and inferior PVs resulting in a figure of 8 lesion set as well as a posterior inferior line allowing for electrical isolation of the posterior left atrial wall. An encircling lesion of the superior vena cava (SVC) directed at electrical isolation of the SVC is also shown. SVC isolation is performed if focal firing from the SVC can be demonstrated. A subset of operators empirically isolates the SVC. D: Some of the most common sites of ablation lesions when complex fractionated electrograms are targeted (these sites are also close to the autonomic GP) (Reproduced with permission from [18])
Several studies have been performed to evaluate the benefits of AF ablation in the systolic heart failure population.
In a small study of patients with atrial fibrillation and systolic heart failure (mean EF 42 %), atrial fibrillation ablation increased LVEF from 42 % +/−9 % to 56+/−8 %, (P <0.001) [20] (Fig. 3.4).
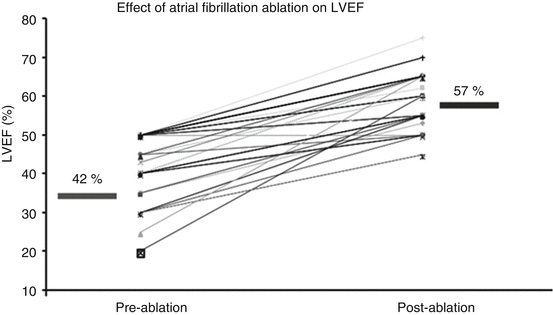

Fig. 3.4
Graph demonstrating effects of AF ablation on left ventricular ejection fraction in patients undergoing atrial fibrillation who have preexisting systolic heart failure. In this population, ablation increased the mean LVEF from 42 % ± 9 % to 56 ± 8 %, P <0.001 (Reproduced with permission from [20])
In another study, 58 patients with LVEF <45 % and NYHA Class II or higher underwent catheter-based radiofrequency ablation; maintenance of sinus rhythm was associated with significant improvement in LV function, exercise capacity, and quality of life [21]. The improvement in EF was highest in patients with inadequate rate control before the ablation (24+/−8 %), highlighting the potential role for tachycardia-mediated cardiomyopathy in this population. The majority of patients remained in sinus rhythm at the 12 months follow-up (69 % without antiarrhythmic drugs and 78 % with antiarrhythmic medications). Interestingly, the success rate of ablation at 12 months was no different between patients with systolic heart failure and patients without systolic heart failure (69 % vs. 71 %, respectively, P = 0.84). Although this study was small and not randomized, compelling evidence exists for the efficacy of catheter ablation in patients with heart failure. It is very important to note that the ablation technique involved additional lines in the left atrium besides pulmonary vein isolation, allowing correction of substrates typically involved in CHF and AF.
Results from a larger nonrandomized trial (94 patients) demonstrated slightly different results [22]. Patients with LVEF <40 % who underwent atrial fibrillation ablation had a higher AF recurrence rate (27 %) than patients with normal LVEF (13 %). Overall, there was no significant increase in EF in patients with systolic heart failure after successful catheter ablation (36 % before ablation to 41 % after ablation, P = 0.1) [22]. One possible reason for the lack of EF improvement in this study despite similar AF ablation success rates as in prior studies is that the patients in this study had better rate control pre-ablation than in prior studies; however, the main difference with the previous study consists in the technique of performing the ablation: Natale’s group focused only on pulmonary vein isolation without additional lines. Among the patients who did show improvements in EF, the average increase in EF was 7 %.
The need for a randomized clinical study was answered by the CAMTAF trial [23] which enrolled 52 patients with persistent AF predominantly and EF below 35 %.
The hypothesis of this trial was that restoration of sinus rhythm with ablation improves LV function and HF symptoms compared to a rate control strategy in patients with AF and heart failure.
The primary endpoint was EF at 6 months follow-up. Of these 50 patients, 26 patients underwent catheter ablation and 24 patients underwent rate control. In the patients undergoing AF ablation, the freedom from AF was 81 % off of antiarrhythmic drugs. The LVEF at baseline was 32+/−8 % in the ablation arm and 34+/−12 % in the rate control arm [23].
The LVEF at 6 months was 39.9 % (CI 35.2 %–44.7 %) in the catheter ablation group compared with 31.0 % (CI, 25.5–36.6 %) in the medical group (P = 0.015). Therefore, the mean increase in EF was 8.1 % in the ablation arm compared with a decrease in 3.6 % in the rate control arm. This improvement remained significant at 12 months. In addition, peak VO2 max and Minnesota living with heart failure score were significantly improved in the catheter ablation as compared with the rate control group [23]. This is the first randomized clinical trial to demonstrate that catheter ablation for AF in patients with heart failure may be a better strategy than rhythm control. However, larger studies should be performed to evaluate important clinical endpoints such as heart failure hospitalization, mortality, and cost-effectiveness.
Finally, a study entitled the Ablation vs. Amiodarone for Treatment of Atrial Fibrillation in Patients with Congestive Heart Failure and Implanted ICD/CRT-D, AATAC-AF in Heart Failure Trial, tested the hypothesis that catheter ablation for persistent AF in patients with HF is superior to amiodarone [24]. This trial enrolled patients with persistent AF, EF <=40 %, and who had either an ICD or CRT-D implant. The presence of the implantable defibrillators allowed for very accurate detection of atrial arrhythmia postablation. Two hundred and three patients were randomized to either catheter ablation or amiodarone. Patients in the catheter ablation group had a 70 % recurrence-free rate, while the amiodarone group had a 34 % recurrence-free rate. Of the 102 patients randomized to AF ablation, the majority (80 patients) underwent combination PVI ablation, posterior wall ablation, and non-pulmonary vein trigger ablation, while 22 patients underwent PVI alone [24]. Of note, the more extensive ablation had a freedom from AF rate of 78.8 %, while patients who had straightforward PVI had a freedom from AF rate of 36.4 % (P <0.001), demonstrating that more extensive ablation beyond PVI had a higher success rate. At 2-year follow-up, the patients who maintained sinus rhythm (N = 105) compared to the patients who had AF recurrence (N = 98) had a significantly lower hospitalization rate, higher ejection fraction improvement, longer 6-min walk distance, and lower Minnesota Living with Heart Failure scores. Finally, all-cause mortality was statistically lower in the AF ablation group as compared to the amiodarone group (8 % vs. 18 %), respectively, (P = 0.032).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


