Fig. 20.1
ECG shows atrial fibrillation with high ventricular rate
ECG showed atrial fibrillation (AF) with high ventricular rate (130 beats per minute). There were diffuse and nonspecific repolarization alterations.
Chest X-Ray (Fig. 20.2)
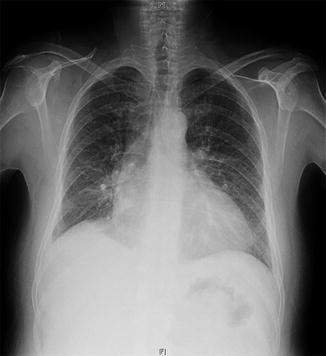
Fig. 20.2
Chest X-ray
A standard chest X-ray demonstrated the presence of bilateral lung interstitial edema and heart enlargement.
The patient was admitted to the cardiology department with the initial diagnosis of heart failure in patient with AF at high ventricular rate.
He was treated with a diuretic (furosemide) and rate-control agents (digoxin and metoprolol) with an improvement of symptoms after 24 h.
Echocardiography was recorded and showed the following findings: “normal-sized left ventricle (60 ml/m2) with severe depression of global systolic function (EF less than 20 %) due to diffuse hypokinesia. Severe dilatation of the left atrium (index volume 40 ml/m2). High filling pressure. Normal right ventricle size with reduced systolic function (TAPSE 12 mm). Moderate dilatation of the right atrium. No pericardial effusion. No gradients. Mild to moderate mitral regurgitation. Moderate to severe tricuspid regurgitation with high pulmonary arterial pressure (40 mmHg)” (Fig. 20.3).
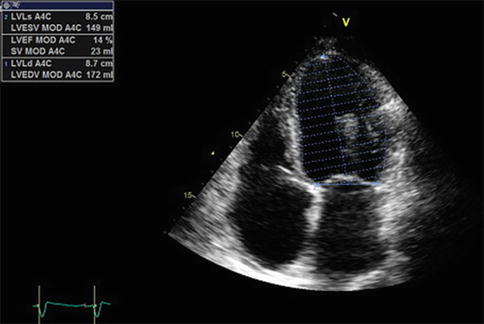

Fig. 20.3
Echocardiography. Severe depression of left ventricular global systolic function. LVEDV left ventricular end-diastolic volume, LVESV left ventricular end-systolic volume, LVEF left ventricular ejection fraction
What Are the Possible Causes of This Severe Depression of Left Ventricular Systolic Function?
Possible alternatives:
Ischemic heart disease
Idiopathic dilated cardiomyopathy
Myocarditis
Tachycardiomyopathy
An invasive coronary angiography was performed in order to exclude a coronary disease (chest pain, elevated myocardial necrosis markers) but didn’t show any coronary lesion (Fig. 20.4a, b).
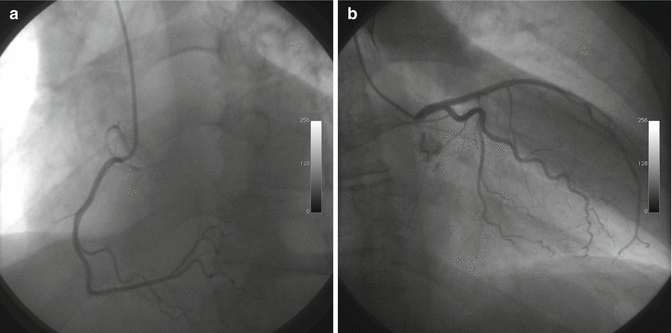

Fig. 20.4
Coronary angiography. Right coronary angiography (a) and left coronary angiography (b)
The patient had heart failure and therefore an electrical cardioversion was indicated.
Because AF was not datable, a transesophageal echocardiography (TEE) was done to exclude atrial thrombi before going to electrical cardioversion. TEE didn’t show atrial thrombi and a successful electrical cardioversion was performed. The sinus rhythm was restored after a single synchronized biphasic shock at 150 J. Amiodarone was there introduced for rhythm control together with warfarin to prevent thromboembolic events (Fig. 20.5).
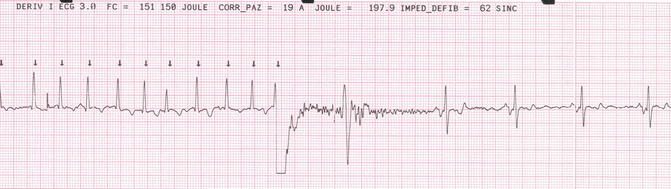

Fig. 20.5
Electrical cardioversion. Synchronized biphasic shock at 150 J restores sinus rhythm
An acute myocarditis was also later excluded by magnetic resonance imaging.
The final diagnosis was “severe biventricular systolic dysfunction secondary to tachycardiomyopathy (high-rate AF).” The patient was dismissed after 3 days in good clinical state with the following medical therapy: amiodarone 200 mg at 8.00 a.m., losartan 25 mg at 8.00 a.m., furosemide 50 mg at 8.00 a.m. and at 6.00 p.m., warfarin at 4.00 p.m. (variable dose to maintain INR from 2.0 to 3.0), eplerenone 25 mg at 6.00 p.m., and metoprolol 50 mg at 8.00 a.m. and at 8.00 a.m.
After 1 month, another echocardiogram was recorded. It showed a great biventricular systolic function improvement (EF 57 %; TAPSE 17 mm) and a reduction of mitral and tricuspid regurgitation (now mild). Also pulmonary arterial pressure returned to normal. ECG confirmed sinus rhythm maintenance (Fig. 20.6a, b).
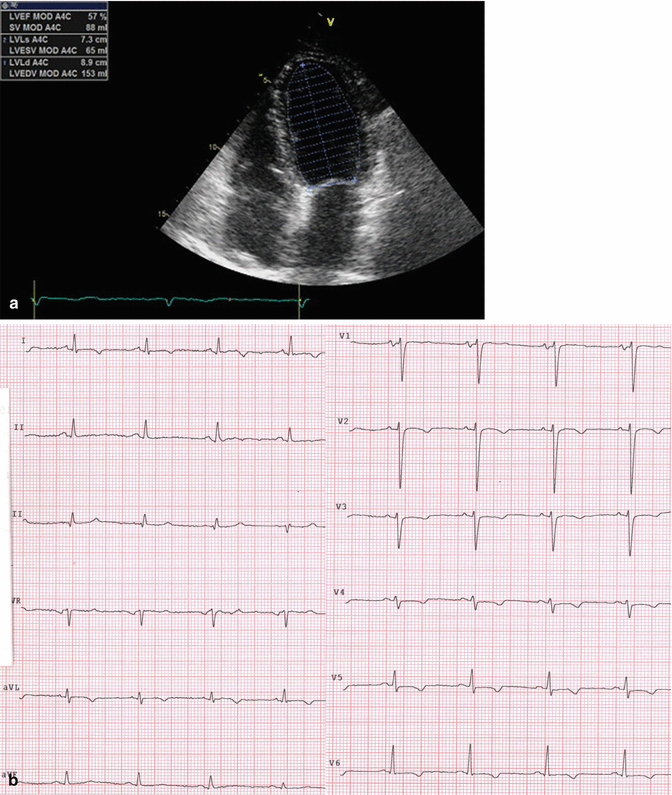

Fig. 20.6
Echocardiography. Normal left ventricular global systolic function (a). ECG with sinus rhythm (b)
The full systolic function recovery consequent of a stable sinus rhythm confirmed the previous diagnosis of tachycardiomyopathy. Warfarin was stopped after 1 month from cardioversion because patient had not any stroke risk factor (CHA2DS2-VASc = 0).
20.2 Atrial Fibrillation (AF)
Definition and Epidemiology
Atrial fibrillation (AF) is a supraventricular tachyarrhythmia characterized by chaotic and irregular atrial electrical activity. It’s the most common sustained arrhythmia in clinical practice and is the leading cause of hospitalization for arrhythmias.
The prevalence of AF in the general population is about 1–2 % and increased with age; 70 % of patients with atrial fibrillation are more than 65 years old [1, 2].
More than six million in Europe have this form of arrhythmia, and its prevalence is expected to double over the next 50 years with the aging population.
Classification
AF is classified according to the modality of presentation, its duration and the possibility of restoration of sinus rhythm into:
1.
First diagnosed AF, in patients presenting with the arrhythmia for the first time
2.
Paroxysmal AF, in general, self-terminating within 48 h, but with a maximum duration up to 1 week
3.
Persistent AF, when an episode of AF lasts more than 7 days or requires cardioversion to restore sinus rhythm
4.
Long-standing persistent AF if AF lasts more than a year when an attempt is made to restore the rhythm
5.
Permanent AF if it is chronic and no more attempts to restore normal heart rhythm will be made [3]
Clinical Presentation and Diagnostic Evaluation
The most common symptoms in patients with AF are palpitations (54 %), which are more common in paroxysmal forms, and dyspnea (44 %), which is prevalent in permanent type. Other common symptoms are easy fatigability and asthenia (14 %), chest pain (10 %), and dizziness and syncope (10 %) [4, 5].
AF may be also asymptomatic and consequently silent; the frequency of silent FA varies depending on the method used for recording, reaching up to 51–74 % if considering the memories of pacemakers and ICD [6, 7].
The high prevalence of asymptomatic AF has implications on the need to start the oral anticoagulant therapy; various trials showed that only episodes of AF long enough (>5–24 h) are associated with an increased risk of thromboembolism [8, 9].
The European Heart Rhythm Association had proposed a new classification for assessing symptoms during AF (EHRA score) [10, 11]: class I, related to no symptoms; class II mild symptom, with no impairment of normal daily activities; class III, severe symptoms that affected normal daily activity; and class IV, disabling symptoms with disruption of normal daily activity.
The diagnosis of AF requires an ECG performed during the arrhythmia. ECG provides information not only on heart rhythm but also on the presence of ventricular hypertrophy, ventricular pre-excitation, bundle branch block, myocardial necrosis, and concomitant arrhythmias.
Transthoracic echocardiography (TTE) is extremely useful for assessing the existence of an underlying heart disease; it helps to define the presence of a valvular disease and its severity to evaluate chamber dimension and left ventricular systolic and diastolic function and estimate pulmonary pressure.
Transesophageal echocardiography (TEE) is used to assess the presence of e.g., left atrial appendage thrombi before cardioversion of nondatable AF.
Chest X-ray is useful in particular in cases of AF associated to symptoms of heart failure for the evaluation of the state of pulmonary circulation. It can reveal the presence of concomitant AF-associated pulmonary diseases (e.g., COPD).
Laboratory tests to be performed are tests for thyroid hormones (TSH, FT4), serum electrolytes, blood counts, and renal and hepatic functions that help in choosing antiarrhythmic and anticoagulant drugs.
ECG monitoring system as ambulatory ECG Holter or external loop recorder may be used in case of palpitations suspicious for paroxysmal AF but without previous electrocardiographic documentation of arrhythmia and for the diagnosis of asymptomatic episodes of AF.
Performing other imaging studies should be evaluated case by case.
Treatment
In AF treatment, we have to consider two aspects: (1) prevention of thromboembolism and (2) treatment of the arrhythmia.
Antithrombotic Therapy
Patients, during prolonged (more than 48 h) AF episode, have to do anticoagulant therapy to reduce cardio embolic stroke risk. Indication to lifelong oral anticoagulant therapy in patients with previous AF episodes (regardless of current rhythm) is based on a stroke risk score (CHA2DS2-VASc score). CHA2DS2-VASc score [congestive heart failure (1 point), hypertension (1 point), age more than 75 (2 points), diabetes (1 point), previous stroke or TIA (2 points), vascular disease (1 point), age more than 65 (1 point), sex category female (1 point)] greater than or equal to 1 identifies patients at risk of stroke who have indication to do lifelong oral anticoagulant therapy. The stroke rate in AF patients increases progressively from 1.3 %/year (CHA2DS2-VASc = 1) to 15.2 %/year (CHA2DS2-VASc = 9). HAS-BLED score [hypertension (1 point), abnormal renal and liver functions (1 point each), previous stroke (1 point), bleeding (1 point), labile INRs (1 point), elderly more than 65 years (1 point), drug or alcohol abuse (1 point each)] is used to assess the bleeding risk but not to contraindicate the oral anticoagulant therapy. When HAS-BLED score is greater than or equal to three, the bleeding risk is high and a close follow-up of anticoagulated patients is necessary.
The oral anticoagulant therapy can be practiced with vitamin K antagonist (warfarin) dosing to obtain INR values from 2.0 to 3.0 or with the new oral anticoagulants (NOAC) (dabigatran, a direct factor II inhibitor; apixaban; rivaroxaban; and edoxaban, a direct factor X inhibitor). NOAC are fixed-dose drugs (twice daily for dabigatran and apixaban, once daily for rivaroxaban and edoxaban) that do not require periodical coagulation assessment; neither the dose has to be varied in response to changes in laboratory coagulation parameter (INR or aPTT). NOAC are not indicated in patients with severe renal impairment (creatinine clearance, assessed by Cockcroft-Gault formula, less than 30 ml/min) and in valvular AF (prosthetic valves and rheumatic valvular disease, in particular mitral stenosis).
In patients with important contraindications to lifelong anticoagulant therapy, it is possible to perform the occlusion of the left atrial appendage (surgical or percutaneous). This is a non-pharmacological method to reduce stroke risk. The rationale of this technique is that the left atrial appendage is the main (but not the only) site of thrombi formation.
Treatment of Arrhythmia
Acute Therapy: In unstable patient, an immediate electrical cardioversion is mandatory. A stable patient with a rapid ventricular response needs an acute therapy to control the ventricular rate (beta-blockers, non-dihydropyridine calcium-channel antagonist, digoxin, or a combination of them). In case of slow ventricular rate AF, a therapy with atropine or temporary pacemaker may be useful in symptomatic patient.
If AF is of recent onset (less than 48 h), it is possible to try to restore sinus rhythm by electrical or pharmacological cardioversion. Electrical cardioversion consists of a synchronized electrical shock delivery in sedated patients, and it is more effective than pharmacological cardioversion. For pharmacological cardioversion, the choice is between class IC antiarrhythmic drugs (flecainide or propafenone for patients without structural heart disease and amiodarone for patient with structural heart disease). Class IC antiarrhythmic drugs can be administered intravenously or with oral high dose (“pill-in-the-pocket” approach), and their efficacy length is about 2–4 h. Pharmacological cardioversion does not require sedation of patient.
If AF onset is prolonged upto 48 h, the cardioversion can be made only after a TEE that excludes left atrium or left atrium appendage thrombi or after at least 3 weeks of correct anticoagulation (INR 2.0–3.0 or constant intake of NOAC). If TEE reveals thrombi, cardioversion is not indicated and a new TEE has to be recorded after at least 3 weeks of correct anticoagulation before cardioversion to assess if thrombi are still present. In this case, long-term oral anticoagulant therapy is indicated regardless of CHA2DS2-VASc score and a rate-control strategy is recommended.
In patients with AF more than 48 h, 4 weeks of anticoagulation therapy is indicated after cardioversion independently from CHA2DS2-VASc score.
Long-Term Therapy: there are two strategies for long-term management of AF, rhythm control and rate control.
Rhythm-control strategy is based on preventing AF recurrence and maintaining sinus rhythm by the administration of antiarrhythmic drugs. There are different antiarrhythmic drugs used to try to maintain sinus rhythm (flecainide, propafenone, sotalol, dronedarone, amiodarone). Class IC antiarrhythmic drugs (flecainide and propafenone) can be safely used to maintain sinus rhythm in patient without structural heart disease. Amiodarone is better than other antiarrhythmic drugs to maintain sinus rhythm (the number of patients needed to be treated is three for amiodarone, four for flecainide, five for propafenone, and eight for sotalol), but it is generally used in patients with AF recurrences despite other drug therapies because it has several side effects (thyroid dysfunctions, vision problems, liver disease, lung disorder, etc.). Dronedarone is less effective than amiodarone, but it can be used in patients experiencing amiodarone toxicity or side effects.
The presence and type of structural heart disease are important for choosing among different antiarrhythmic drugs (Fig. 20.7).
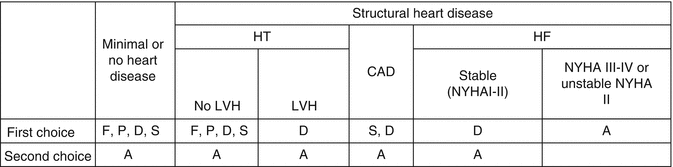





Fig. 20.7
Antiarrhythmic drugs for rhythm control. HT hypertension, CAD coronary artery disease, HF heart failure, F flecainide, P propafenone, D dronedarone, S sotalol, A amiodarone
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


