Atrial Fibrillation and Flutter
Atrial fibrillation (AF) is the most common sustained arrhythmia seen in clinical practice. There are estimated to be more than 2 million patients with AF in the United States. The prevalence and incidence of AF increase with advancing age. The mainstay of therapy includes pharmacologic rate control and antiarrhythmic therapy, cardioversion, and antithromboembolic management. Non-pharmacologic therapies, including ablation, device, and surgical approaches, are also becoming increasingly utilized.
EPIDEMIOLOGY
Prevalence
 0.4% general population
0.4% general population
 0.2% in population 25 to 34 years old
0.2% in population 25 to 34 years old
 2% to 5% in population >60 years old
2% to 5% in population >60 years old
 18% in population >85 years old
18% in population >85 years old
 8% to 14% in hospitalized patients
8% to 14% in hospitalized patients
Incidence
 The incidence of AF increases from <0.1% per year (>160,000 new US cases year) in those under 40 years of age to 1.5% per year in females and 2% per year in males over the age of 80 (Kannel et al. 1983).
The incidence of AF increases from <0.1% per year (>160,000 new US cases year) in those under 40 years of age to 1.5% per year in females and 2% per year in males over the age of 80 (Kannel et al. 1983).
 20% to 40% after cardiac surgery
20% to 40% after cardiac surgery
FACTORS PREDISPOSING TO ATRIAL FIBRILLATION
The most common cardiovascular (CV) diseases associated with AF are hypertension and ischemic heart disease. Other predisposing conditions include:
 Advancing age
Advancing age
 Rheumatic heart disease (especially mitral valve disease)
Rheumatic heart disease (especially mitral valve disease)
 Nonrheumatic valvular disease
Nonrheumatic valvular disease
 Cardiomyopathies
Cardiomyopathies
 Congestive heart failure (CHF)
Congestive heart failure (CHF)
 Congenital heart disease
Congenital heart disease
 Sick sinus syndrome/degenerative conduction system disease
Sick sinus syndrome/degenerative conduction system disease
 Wolff–Parkinson–White syndrome
Wolff–Parkinson–White syndrome
 Pericarditis
Pericarditis
 Pulmonary embolism
Pulmonary embolism
 Thyrotoxicosis
Thyrotoxicosis
 Chronic lung disease
Chronic lung disease
 Neoplastic disease
Neoplastic disease
 Postoperative states
Postoperative states
 Diabetes
Diabetes
 Normal hearts affected by high adrenergic states, alcohol, stress, drugs (especially sympathomimetics), excessive caffeine, hypoxia, hypokalemia, hypoglycemia, or systemic infection
Normal hearts affected by high adrenergic states, alcohol, stress, drugs (especially sympathomimetics), excessive caffeine, hypoxia, hypokalemia, hypoglycemia, or systemic infection
MORBIDITY AND MORTALITY
Survival
The presence of AF leads to a 1.5- to 2-fold increase in total and CV mortality (Emelia et al., 1998). Factors that may increase mortality include:
 Age
Age
 Mitral stenosis
Mitral stenosis
 Aortic valve disease
Aortic valve disease
 Coronary artery disease (CAD)
Coronary artery disease (CAD)
 Hypertension
Hypertension
 CHF
CHF
Patients with myocardial infarction (MI) or CHF have higher mortality if AF is present.
Stroke/Thromboembolism
AF predisposes to stroke and thromboembolism.
 Five- to sixfold increased risk of stroke (17-fold with rheumatic heart disease [RHD])
Five- to sixfold increased risk of stroke (17-fold with rheumatic heart disease [RHD])
 3% to 5% per year rate of stroke in nonvalvular AF
3% to 5% per year rate of stroke in nonvalvular AF
 Single major cause (50%) of cardiogenic stroke
Single major cause (50%) of cardiogenic stroke
 75,000 strokes per year
75,000 strokes per year
 Silent cerebral infarction risk
Silent cerebral infarction risk
 Risk increases with age, concomitant CV disease, and stroke risk factors
Risk increases with age, concomitant CV disease, and stroke risk factors
Tachycardia-Induced Cardiomyopathy
Persistent rapid ventricular rates can lead to tachycardia-mediated cardiomyopathy and left ventricular (LV) systolic dysfunction. These are, however, reversible with ventricular rate control and regularization. Control can be achieved with medical rate control, atrioventricular (AV) node ablation, or achievement of sinus rhythm (SR). An atrial cardiomyopathy may develop leading to structural remodeling with an increase in atrial size.
Symptoms and Hemodynamics
 Rapid ventricular rates
Rapid ventricular rates
 Irregularity of ventricular rhythm
Irregularity of ventricular rhythm
 Loss of AV synchrony
Loss of AV synchrony
 Symptoms: limitation in functional capacity, palpitations, fatigue, dyspnea, angina, CHF
Symptoms: limitation in functional capacity, palpitations, fatigue, dyspnea, angina, CHF
PATHOGENESIS
While the pathophysiology of AF remains incompletely understood, it has been shown that AF requires a trigger and a substrate to sustain reentry. The triggering mechanism in most patients comes from ectopic firing within the pulmonary veins into which sleeves of atrial myocardium extend. Once AF has been sustained for a period of time, electrical and structural changes take place within the atria that can convert transient AF to persistent AF. Electrical changes, such as shortening of the atrial refractory period, occur shortly after AF onset and are reversible with conversion back to SR. Structural changes may take longer to develop, however, and are less amenable to reversal. In patients with CHF, the pathophysiology of AF is somewhat different. In this patient population, areas of interstitial fibrosis are found within the atria that lead to heterogeneous electrical conduction. These areas of slowed electrical conduction predispose to the development of AF.
 Electrical activation: rapid, multiple waves of depolarization with continuously changing, wandering pathways
Electrical activation: rapid, multiple waves of depolarization with continuously changing, wandering pathways
 Intracardiac electrograms: irregular, rapid depolarizations, often >300 to 400 beats/min (bpm)
Intracardiac electrograms: irregular, rapid depolarizations, often >300 to 400 beats/min (bpm)
 Mechanical effects:
Mechanical effects:
 Loss of coordinated atrial contraction
Loss of coordinated atrial contraction
 Irregular electrical inputs to the AV node and His–Purkinje system leading to irregular ventricular contraction
Irregular electrical inputs to the AV node and His–Purkinje system leading to irregular ventricular contraction
 Surface electrocardiogram:
Surface electrocardiogram:
 No discrete P waves
No discrete P waves
 Irregular fibrillatory waves
Irregular fibrillatory waves
 Irregularly, irregular ventricular response
Irregularly, irregular ventricular response
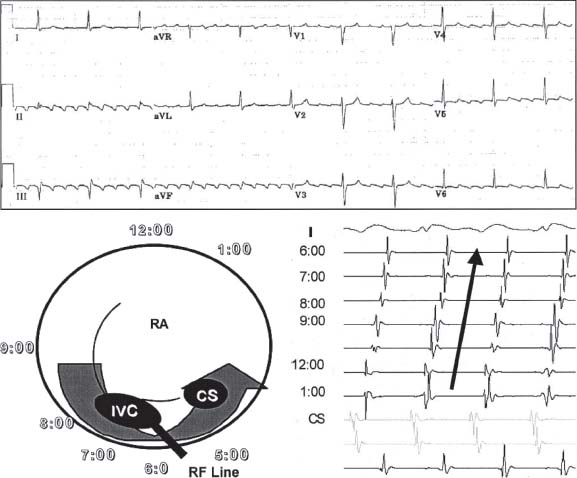
Atrial Flutter Reentrant Mechanism
Cavotricuspid Isthmus-Dependent Atrial Flutter
 Cavotricuspid isthmus (CTI)-dependent flutters refers to circuits, which involve the isthmus of tissue in the right atrium between the tricuspid annulus and inferior vena cava (IVC) (Fig. 28.1).
Cavotricuspid isthmus (CTI)-dependent flutters refers to circuits, which involve the isthmus of tissue in the right atrium between the tricuspid annulus and inferior vena cava (IVC) (Fig. 28.1).
 The circuit can propagate around the isthmus in a clockwise or counterclockwise direction.
The circuit can propagate around the isthmus in a clockwise or counterclockwise direction.
 Counterclockwise atrial flutter is characterized by dominant negative flutter waves in the inferior leads and positive flutter deflection in lead V1.
Counterclockwise atrial flutter is characterized by dominant negative flutter waves in the inferior leads and positive flutter deflection in lead V1.
 Clockwise atrial flutter is characterized by positive flutter waves in inferior leads and negative flutter waves in lead V1.
Clockwise atrial flutter is characterized by positive flutter waves in inferior leads and negative flutter waves in lead V1.
 In contrast to coarse AF, the flutter waves on an ECG will usually have the same morphology, amplitude, and cycle length.
In contrast to coarse AF, the flutter waves on an ECG will usually have the same morphology, amplitude, and cycle length.
 Ablation of the CTI is curative.
Ablation of the CTI is curative.
Noncavotricuspid Isthmus-Dependent Atrial Flutter
 Noncavotricuspid isthmus (NCTI)-dependent flutters do not use the CTI. NCTI flutters are often related to atrial scar which creates a conduction block and a central obstacle that allows for reentry.
Noncavotricuspid isthmus (NCTI)-dependent flutters do not use the CTI. NCTI flutters are often related to atrial scar which creates a conduction block and a central obstacle that allows for reentry.
 NCTI can be found in patients with prior cardiac surgery involving the atrium, such as repair of congenital heart disease, mitral valve surgery, or maze procedure as well as in patients post pulmonary vein isolation procedures.
NCTI can be found in patients with prior cardiac surgery involving the atrium, such as repair of congenital heart disease, mitral valve surgery, or maze procedure as well as in patients post pulmonary vein isolation procedures.
 NCTI-dependent flutters are less common than CTI flutters.
NCTI-dependent flutters are less common than CTI flutters.
Treatment
 Atrial flutter may be difficult to treat medically (it is notoriously difficult to rate control) and may develop with organization of AF reentrant flutter circuits during treatment with antiarrhythmic therapy.
Atrial flutter may be difficult to treat medically (it is notoriously difficult to rate control) and may develop with organization of AF reentrant flutter circuits during treatment with antiarrhythmic therapy.
 Successful ablation is dependent on identifying a critical portion of the reentry circuit where it can be interrupted with catheter ablation.
Successful ablation is dependent on identifying a critical portion of the reentry circuit where it can be interrupted with catheter ablation.
ATRIAL FIBRILLATION DEFINITIONS
 Lone: Patients under the age of 60 years with absence of cardiopulmonary or other conditions predisposing to AF
Lone: Patients under the age of 60 years with absence of cardiopulmonary or other conditions predisposing to AF
 New Onset: First episode of AF
New Onset: First episode of AF
 Recurrent: Has two or more paroxysmal or persistent episodes
Recurrent: Has two or more paroxysmal or persistent episodes
 Paroxysmal: Self-terminating within 7 days, generally lasting 24 hours
Paroxysmal: Self-terminating within 7 days, generally lasting 24 hours
 Persistent: Is not self-terminating within 7 days or is terminated with treatment
Persistent: Is not self-terminating within 7 days or is terminated with treatment
 Permanent: Persistent despite cardioversion
Permanent: Persistent despite cardioversion
EVALUATION
History
 Precipitating factors and conditions
Precipitating factors and conditions
 Alcohol, caffeine, sympathomimetics, herbal supplements, or other drug use
Alcohol, caffeine, sympathomimetics, herbal supplements, or other drug use
 Duration and frequency of episodes
Duration and frequency of episodes
 Degree of associated symptoms
Degree of associated symptoms
 Manner of AF initiation
Manner of AF initiation
 Prior therapies for AF (past antiarrhythmic drugs that may have failed or past ablation attempts)
Prior therapies for AF (past antiarrhythmic drugs that may have failed or past ablation attempts)
Documentation of Atrial
Fibrillation and Initiation
 ECGs, rhythm strips
ECGs, rhythm strips
 Transtelephonic (remote) event monitoring
Transtelephonic (remote) event monitoring
 Evaluation for precipitating bradycardia, paroxysmal supraventricular tachycardia (PSVT), atrial flutter, atrial ectopy, atrial tachycardia
Evaluation for precipitating bradycardia, paroxysmal supraventricular tachycardia (PSVT), atrial flutter, atrial ectopy, atrial tachycardia
Diagnostic Testing
 Lab studies—thyroid function, renal, and hepatic tests
Lab studies—thyroid function, renal, and hepatic tests
 Echocardiogram—evaluate LV function, valves, atrial size
Echocardiogram—evaluate LV function, valves, atrial size
 Functional stress testing or cardiac catheterization—evaluate for CAD in patients with risk factors and evaluate candidacy for 1C agents
Functional stress testing or cardiac catheterization—evaluate for CAD in patients with risk factors and evaluate candidacy for 1C agents
MANAGEMENT OF ATRIAL FIBRILLATION
Treatment Strategies
 Ventricular rate control
Ventricular rate control
 AV nodal–blocking drugs
AV nodal–blocking drugs
 Atrioventricular node (AVN) modification/ablation and pacing
Atrioventricular node (AVN) modification/ablation and pacing
 Achievement and maintenance of SR
Achievement and maintenance of SR
 Antiarrhythmic drugs
Antiarrhythmic drugs
 Cardioversions
Cardioversions
 Nonpharmacologic therapies
Nonpharmacologic therapies
– Ablation
– Surgery—Maze procedure
 Anticoagulation
Anticoagulation
Atrial Fibrillation Follow-Up Investigation of Rhythm Management
The Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study (Wyse et al., 2002) was a multicenter trial of rate versus rhythm control strategies (Table 28.1). It tested the hypothesis that in patients with AF, total mortality with primary therapy intended to maintain SR is equal to that with primary therapy intended to control heart rate. The study randomized 4,060 patients (>65 years old or with risk factors for stroke), with a primary endpoint of total mortality. No significant difference in total mortality was found among strategies, although there was a strong trend toward better survival in the rate-control arm. The study also showed that continued anticoagulation is important even in the rhythm-control arm, so this may be the best strategy in relatively asymptomatic older patients with good rate control.
TABLE
28.1 Rate Control versus Rhythm Control

Control of Ventricular Rate
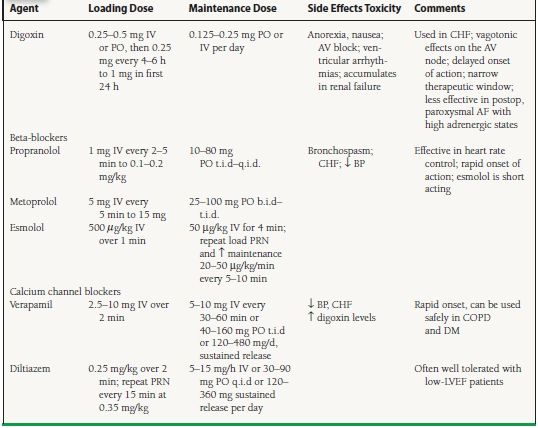
Rapid ventricular rates can cause symptoms and/or ventricular dysfunction. The goal of treatment, a heart rate of 70 to 100 bpm at rest, can be achieved pharmacologically with agents that slow AV nodal conduction, such as digoxin, beta-adrenergic blockers, and calcium channel blockers (Table 28.2). These agents, however, should not be used in patients with ventricular preexcitiation due to the risk of very rapid antidromic conduction during AF over the pathway. In patients who are hemodynamically stable with evidence of pre-excited AF, amiodarone, ibutilide, procainamide, or disopyramide are acceptable choices.
TABLE
28.2 Pharmacologic Rate Control for Atrial Arrhythmias
The RACE II trial compared strict rate control (resting heart rate <80 bpm) to lenient rate control (resting heart rate<110 bpm) in patients with permanent AF. Lenient rate control was comparable to strict rate control in terms of reaching the components of the primary endpoint. In addition, lenient rate control was much easier to achieve compared to strict rate control.
Digoxin
Digoxin has direct and indirect effects on the AV node, with a primary vagotonic effect. Advantages include:
 It is inexpensive.
It is inexpensive.
 It can be given intravenously
It can be given intravenously
 It can be used safely in patients with heart failure.
It can be used safely in patients with heart failure.
 It is effective in controlling resting ventricular rates in chronic, persistent AF.
It is effective in controlling resting ventricular rates in chronic, persistent AF.
Disadvantages are that:
 Peak onset of heart rate-lowering effect is delayed by 1 to 4 hours.
Peak onset of heart rate-lowering effect is delayed by 1 to 4 hours.
 The therapeutic window is narrow.
The therapeutic window is narrow.
 It is less effective in rate control of paroxysmal AF and should never be used as the sole agent for rate control in these patients.
It is less effective in rate control of paroxysmal AF and should never be used as the sole agent for rate control in these patients.
 It is less effective for rapid rates during hyperadrenergic states, when vagal tone is low, for example, during exercise or in acute MI and ICU settings, because of increased sympathetic tone.
It is less effective for rapid rates during hyperadrenergic states, when vagal tone is low, for example, during exercise or in acute MI and ICU settings, because of increased sympathetic tone.
Digoxin should be used with caution in elderly patients and in patients with decreased renal function.
Beta-Adrenergic Blockers
Advantages of beta-adrenergic blockers are that they:
 Are very effective for heart rate control, even with exercise
Are very effective for heart rate control, even with exercise
 Can be given intravenously
Can be given intravenously
 Have rapid onset of action
Have rapid onset of action
 Have long-term benefits in patients with LV dysfunction
Have long-term benefits in patients with LV dysfunction
Disadvantages of beta-adrenergic blockers are that they:
 May provoke bronchospasm
May provoke bronchospasm
 Are negatively inotropic and may exacerbate CHF
Are negatively inotropic and may exacerbate CHF
 May reduce exercise tolerance as a result of their negative inotropy and chronotropy
May reduce exercise tolerance as a result of their negative inotropy and chronotropy
Calcium Channel Blockers
The advantages of calcium channel blockers such as verapamil and diltiazem include:
 Intravenous availability
Intravenous availability
 Rapid onset of action
Rapid onset of action
 Can be used safely in chronic obstructive pulmonary disease (COPD) and diabetes mellitus
Can be used safely in chronic obstructive pulmonary disease (COPD) and diabetes mellitus
Disadvantages include:
 Negative inotropic effects
Negative inotropic effects
 Can cause hypotension
Can cause hypotension
 Long-term safety questioned
Long-term safety questioned
Class I or III Antiarrhythmic Drugs
Sotalol, dronedarone, amiodarone, propafenone, and flecainide can contribute to ventricular rate control.
NONPHARMACOLOGIC RATE CONTROL
Complete AV Junction Ablation
Radiofrequency catheter ablation of the AV node is usually technically easy to accomplish. It is best used in cases of atrial arrhythmias refractory to standard therapies in highly symptomatic patients.
 Advantages
Advantages
 Effectively controls rapid ventricular rates
Effectively controls rapid ventricular rates
 Significant symptomatic relief and improvement in quality of life demonstrated
Significant symptomatic relief and improvement in quality of life demonstrated
 Can reverse tachycardia-mediated cardiomyopathy
Can reverse tachycardia-mediated cardiomyopathy
 Disadvantages
Disadvantages
 Requires a permanent, rate-responsive pacemaker
Requires a permanent, rate-responsive pacemaker
 The patient is pacemaker dependent.
The patient is pacemaker dependent.
 Pacing RV alone may significantly worsen ventricular function. Biventricular pacing may be considered in patients with impaired LV systolic function.
Pacing RV alone may significantly worsen ventricular function. Biventricular pacing may be considered in patients with impaired LV systolic function.
RESTORATION OF SINUS RHYTHM
Electrical Cardioversion
Electrical cardioversion is the most effective method of restoring SR. In this technique, a shock is synchronized to the R wave. The optimal patch positioning is anterior–posterior (e.g., right parasternal to left paraspinal). For standard monophasic external cardioversion, usual initial energies are 200 J for AF and 50 to 100 J for atrial flutter. Energies can be increased up to 300 J if initial efforts are unsuccessful. Biphasic external conversion, however, requires less energy as a rule. All electrical cardioversion requires sedation with a short-acting anesthetic such as etomidate or methohexital, which is one limitation, compared to pharmacologic cardioversion.
Cardioversion is urgently indicated for patients with clinical instability (e.g., hypotension, ischemia, CHF). It is electively indicated for patients who remain in symptomatic AF after a trial of pharmacologic therapy. Electrical cardioversion is contraindicated in patients with AF and digoxin toxicity or hypokalemia.
Pharmacologic Conversion
A small, randomized, controlled study showed no effect of digoxin on conversion rate. However, quinidine, procainamide, flecainide, propafenone, sotalol, amiodarone, dofetilide, and ibutilide have shown success rates of 31% to 90%. Procainamide, ibutilide, and amiodarone are available for intravenous administration.
Procainamide is usually administered at a dose of 10 to 15 mg/kg IV at ≤50 mg/min, then at 1 to 2 mg/min. It is necessary to monitor blood pressure, as hypotension may require slowing the infusion rate; hemodynamic effects may limit dosing in severe LV dysfunction. It is also necessary to monitor for proarrhythmia—QT prolongation and torsades de pointes. Note that the active metabolite, N-acetyl procainamide (NAPA), may accumulate to toxic levels and cause renal failure.
Ibutilide is a class III potassium channel–blocking agent. In one study, it was shown to be more efficacious than procainamide in converting short-term AF/flutter to SR. Usual dosing is 1 mg IV over 10 minutes, which can be repeated after another 10 minutes. One should monitor for QT prolongation and torsades de pointes.
Amiodarone in its IV form is useful for patients who cannot take oral medications, though it is more expensive. It may be helpful for hemodynamically unstable patients with recurrent AF despite cardioversion or other antiarrhythmic drugs, for whom rate control is refractory to conventional
AV nodal–blocking drugs, or who are intolerant of standard antiarrhythmic or rate-controlling drugs as a result of negative inotropy. Rapid oral loading of amiodarone can usually also be achieved in patients with intact gastrointestinal function (Table 28.3).
TABLE
28.3 Pharmacologic Conversion Regimens