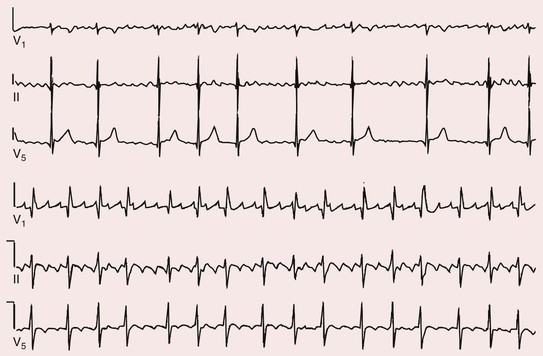
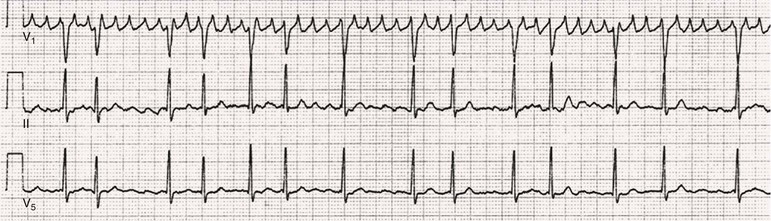
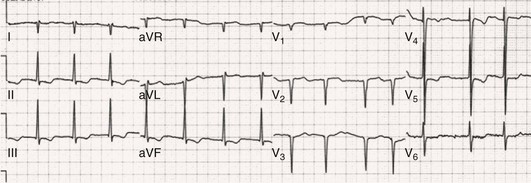
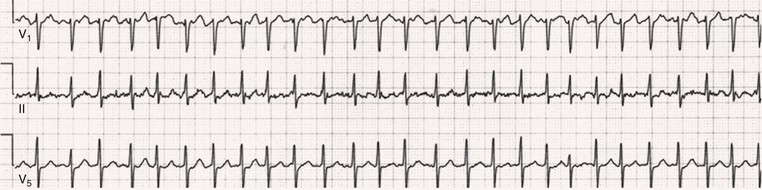
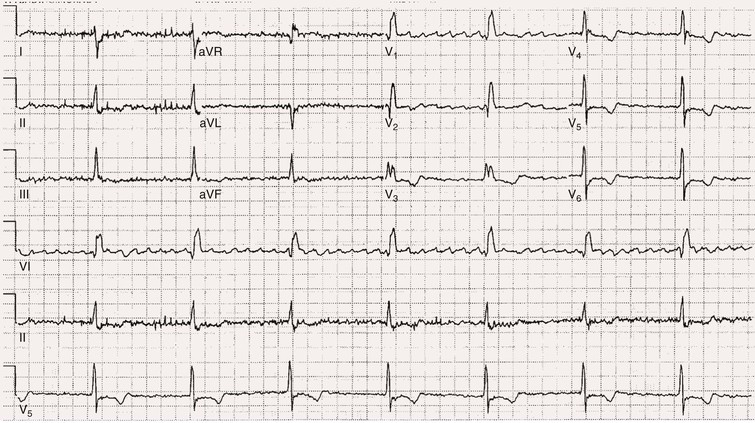
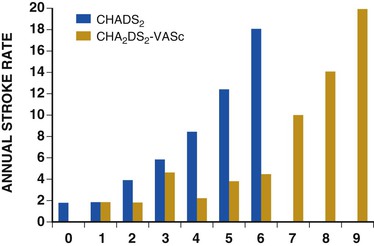
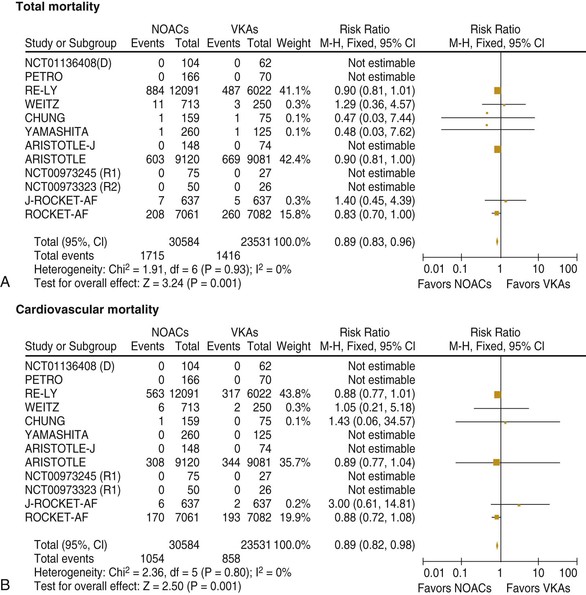
Fred Morady, Douglas P. Zipes Atrial fibrillation (AF) is a supraventricular arrhythmia characterized electrocardiographically by low-amplitude baseline oscillations (fibrillatory or f waves) and an irregularly irregular ventricular rhythm. The f waves have a rate of 300 to 600 beats/min and are variable in amplitude, shape, and timing. In contrast, flutter waves have a rate of 250 to 350 beats/min and are constant in timing and morphology (Fig. 38-1). In lead V1, f waves sometimes appear uniform and can mimic flutter waves (Fig. 38-2). The distinguishing feature from atrial flutter is the absence of uniform and regular atrial activity in other leads of the electrocardiogram. In some patients, f waves are very small and not perceptible on the electrocardiogram. In such patients the diagnosis of AF is based on the irregularly irregular ventricular rhythm (Fig. 38-3). The ventricular rate during AF in the absence of negative dromotropic agents is typically 100 to 160 beats/min. In patients with Wolff-Parkinson-White syndrome, the ventricular rate during AF can exceed 250 beats/min because of conduction over the accessory pathway (see Chapter 37). When the ventricular rate during AF is very rapid (>170 beats/min), the degree of irregularity is attenuated and the rhythm can seem regular (Fig. 38-4). The ventricular rhythm can be regular during AF in patients with a ventricular pacemaker who are fully paced and when a third-degree atrioventricular (AV) block with a regular escape rhythm is present (Fig. 38-5). In these cases the diagnosis of AF is based on the presence of f waves. When there is a third-degree AV block with a junctional escape, a Wenckebach exit block in the AV junction (as can occur during digitalis toxicity) results in a regularly irregular ventricular rate (see Chapters 34 and 37). AF that terminates spontaneously within 7 days is termed paroxysmal, and AF present continuously for more than 7 days is called persistent. AF that is persistent for longer than 1 year is termed longstanding, whereas longstanding AF refractory to cardioversion is termed permanent. However, “permanent AF” is not necessarily permanent in the literal sense because it may be eliminated successfully by surgical or catheter ablation. Some patients with paroxysmal AF can occasionally have episodes that are persistent, and vice versa. The predominant form of AF determines how it should be categorized. A confounding factor in the classification of AF is cardioversion and antiarrhythmic drug therapy. For example, if a patient undergoes transthoracic cardioversion 24 hours after the onset of AF, it is unknown whether the AF would have persisted for more than 7 days. Furthermore, antiarrhythmic drug therapy may change persistent AF into paroxysmal AF. It is generally thought that the classification of AF should not be altered on the basis of the effects of electrical cardioversion or antiarrhythmic drug therapy. Lone AF refers to AF that occurs in patients younger than 60 years who do not have hypertension or any evidence of structural heart disease. This designation is clinically relevant because patients with lone AF are at lower risk for thromboembolic complications, thus eliminating the necessity for anticoagulation with warfarin. In addition, the absence of structural heart disease allows the safe use of rhythm-control drugs such as flecainide in patients with lone AF. Paroxysmal AF can also be classified clinically on the basis of the autonomic setting in which it most often occurs. Approximately 25% of patients with paroxysmal AF have vagotonic AF, in which AF is initiated in the setting of high vagal tone, typically in the evening when the patient is relaxing or during sleep. Drugs that have a vagotonic effect (such as digitalis) can aggravate vagotonic AF, and drugs that have a vagolytic effect (such as disopyramide) may be particularly appropriate for prophylactic therapy. Adrenergic AF occurs in approximately 10% to 15% of patients with paroxysmal AF in the setting of high sympathetic tone, for example, during strenuous exertion. In patients with adrenergic AF, beta blockers not only provide rate control but can also prevent the onset of AF. Most patients have a mixed or random form of paroxysmal AF, with no consistent pattern of onset. AF is the most common arrhythmia treated in clinical practice and the most common arrhythmia for which patients are hospitalized; approximately 33% of arrhythmia-related hospitalizations are for AF. AF is associated with an approximately fivefold increase in the risk for stroke and a twofold increase in the risk for all-cause mortality.1 AF is also associated with the development of heart failure. Estimates of the actual number of individuals with AF in the United States range between 2.3 and 5 million in most studies. The incidence of AF is age and sex related and ranges from 0.1% per year before the age of 40 years to higher than 1.5% per year in women and higher than 2% per year in men older than 80 years. Heart failure, aortic and mitral valve disease, left atrial enlargement, hypertension, and advanced age are independent risk factors for the development of AF, as are obesity and obstructive sleep apnea2 (see Chapter 75). Another risk factor is psoriasis, which when severe, triples the risk for AF in patients younger than 50 years.3 A community-based cohort study in Olmstead County, Minnesota, reported that the age-adjusted incidence of AF per 1000 person-years increased significantly between 1980 and 2000 from 4.4 to 5.4 in men and from 2.4 to 2.8 in women.4 There was a relative increase of 0.6% per year in the age-adjusted incidence of AF. An increase in obesity accounted for 60% of the age-adjusted increase in AF incidence. The number of patients with AF in the United States was estimated to be 3.2 million in 1980 and 5.1 million in 2000 and was projected to be 12.1 to 15.9 million in 2050, all of which are higher than previous estimates. The mechanisms responsible for AF are complex. Triggering events may differ from maintenance mechanisms. In addition, the clinical phenotypes of paroxysmal, persistent, and longstanding persistent AF have different electrophysiologic characteristics because of remodeling and different clinical modulators that affect the substrate, such as heart failure, atrial stretch and ischemia, sympathovagal influences, inflammation, and fibrosis. There are probably two electrophysiologic mechanisms of AF: one or more automatic, triggered, or microreentrant foci, so-called drivers, which fire at rapid rates and cause fibrillation-like activity, and multiple reentrant circuits meandering throughout the atria that annihilate and reform wavelets, thereby perpetuating the fibrillation. In many studies the left atrium contains the site of dominant frequency discharge, with a left-to-right gradient. Both mechanisms may be present simultaneously. In a recent study, computational maps were obtained in patients by signal processing of multiple electrograms recorded simultaneously during AF.5 This technique can reveal electrical rotors and focal sources. A mean of 2.1 sources was found in 97% of 101 patients, with 70% being rotors and 30% being focal sources. Rapid discharges from the pulmonary veins are the most common triggers of AF and may also play a perpetuating role, more so in paroxysmal AF than in persistent AF. This is why pulmonary vein isolation is particularly effective for elimination of paroxysmal AF. In persistent AF, changes in the atrial substrate, including interstitial fibrosis, which contributes to slow, discontinuous, and anisotropic conduction, may give rise to complex fractionated atrial electrograms (CFAEs) and reentry. Therefore, pulmonary vein isolation is rarely sufficient to eliminate persistent AF, and additional ablation of the atrial substrate is usually necessary. Several mutations that are responsible for familial AF and that predispose to AF have been identified.6 These mutations cause a gain of function of repolarization potassium currents that results in shortening of atrial refractoriness and facilitation of atrial reentry. Multiple polymorphisms that are associated with AF that is idiopathic, are associated with structural heart disease, or occur postoperatively have also been identified.6 These polymorphisms are in genes that affect potassium and sodium channels, sarcolipin, the renin-angiotensin system, connexin 40, endothelial nitric oxide synthase, and interleukin-10. The end results are changes in calcium handling, fibrosis, conduction, and inflammation that predispose to AF. Most patients with AF have hypertension (usually with left ventricular hypertrophy; see Chapters 43 and 44) or some other form of structural heart disease. In addition to hypertensive heart disease, the most common cardiac abnormalities associated with AF are ischemic heart disease, mitral valve disease, hypertrophic cardiomyopathy, and dilated cardiomyopathy. Less common causes of AF are restrictive cardiomyopathies such as amyloidosis, constrictive pericarditis, and cardiac tumors. Severe pulmonary hypertension is often associated with AF. Obesity and obstructive sleep apnea (see Chapter 75) are associated with each other, and both have been found to independently increase the risk for AF.2 The data available suggest that atrial dilation and an increase in systemic inflammatory factors are responsible for the relationship between obesity and AF. Possible mechanisms of AF in patients with sleep apnea include hypoxia, surges in autonomic tone, and hypertension. AF can be due to causes that are temporary or reversible. The most common temporary causes are excessive alcohol intake (holiday heart), open heart or thoracic surgery, myocardial infarction, pericarditis (see Chapter 71), myocarditis, and pulmonary embolism (see Chapter 73). The most common correctable cause is hyperthyroidism (see Chapter 81). AF is sometimes induced by tachycardia. Patients with tachycardia-induced AF most often have AV nodal reentrant tachycardia or a tachycardia related to Wolff-Parkinson-White syndrome that degenerates into AF. If a patient with AF has a history of rapid and regular palpitations before the onset of irregular palpitations or has a Wolff-Parkinson-White electrocardiographic pattern, this should raise suspicion for tachycardia-induced AF. Treatment of the tachycardia that triggers the AF often but not always prevents recurrences of AF. The symptoms of AF vary widely between patients and range from none to severe and functionally disabling. The most common symptoms of AF are palpitations, fatigue, dyspnea, effort intolerance, and lightheadedness. Polyuria can occur because of the release of atrial natriuretic hormone. Many patients with symptomatic paroxysmal AF also have asymptomatic episodes, and some patients with persistent AF have symptoms only intermittently, thus making it difficult to accurately assess the frequency and duration of AF on the basis of symptoms. It is estimated that approximately 25% of patients with AF are asymptomatic, more commonly elderly patients and those with persistent AF. Such patients are sometimes erroneously classified as having asymptomatic AF despite the presence of fatigue or effort intolerance. Because fatigue is a nonspecific symptom, it may not be clearly due to persistent AF. “Diagnostic cardioversion” may be helpful by maintaining sinus rhythm for at least a few days to determine whether a patient feels better in sinus rhythm. This can provide a basis to pursue a rhythm-control versus rate-control strategy. Syncope is an uncommon symptom of AF. It can be caused by a long sinus pause on termination of AF in a patient with sick sinus syndrome. Syncope can also occur during AF with a rapid ventricular rate either because of neurocardiogenic (vasodepressor) syncope that is triggered by the tachycardia or because of a severe drop in blood pressure secondary to a reduction in cardiac output. Asymptomatic or minimally symptomatic AF patients are not prompted to seek medical care and can initially be seen with a thromboembolic complication such as stroke or the insidious onset of heart failure symptoms that eventually results in florid congestive heart failure. The hallmark of AF on physical examination is an irregularly irregular pulse. Short R-R intervals during AF do not allow adequate time for left ventricular diastolic filling, which results in a low stroke volume and the absence of palpable peripheral pulse. This leads to a “pulse deficit,” during which the peripheral pulse is not as rapid as the apical rate. Other manifestations of AF on physical examination are irregular jugular venous pulsations and variable intensity of the first heart sound. In a patient who describes irregular or rapid palpitations suggestive of paroxysmal AF, ambulatory monitoring is useful to document whether AF is responsible for the symptoms. If the symptoms occur on a daily basis, a 24-hour Holter recording is appropriate. However, extended monitoring for 2 to 4 weeks with an event monitor or by mobile cardiac outpatient telemetry is appropriate for patients whose symptoms are sporadic (see Chapter 34). The history should be directed at determination of the type and severity of symptoms, the first onset of AF, whether the AF is paroxysmal or persistent, triggers for AF, whether the episodes are random or occur at particular times (such as during sleep), and the frequency and duration of episodes. When it is unclear from the history, 2 to 4 weeks of ambulatory monitoring with an autotrigger event monitor or by mobile cardiac outpatient telemetry is useful to determine whether the AF is paroxysmal or persistent and to quantitate the AF burden in patients with paroxysmal AF. The history also should be directed at identification of potentially correctible causes (e.g., hyperthyroidism, excessive alcohol intake), structural heart disease, and comorbid conditions. Laboratory testing should include thyroid function tests, liver function tests, and renal function tests. Echocardiography is always appropriate to evaluate atrial size and left ventricular function and to look for left ventricular hypertrophy, congenital heart disease (see Chapter 62), and valvular heart disease. Chest radiography is appropriate if the history or findings on physical examination are suggestive of pulmonary disease (see Chapter 15). A stress test is appropriate for evaluation of ischemic heart disease in at-risk patients (see Chapter 13). A major goal of therapy in patients with AF is to prevent thromboembolic complications such as stroke. It is well established that warfarin is more effective than aspirin for prevention of thromboembolic complications.7 However, because of the risk for hemorrhage during warfarin therapy, its use should be limited to patients whose risk for thromboembolic complications is greater than their risk for hemorrhage. Therefore, it is useful to risk-stratify patients with AF to identify appropriate candidates for warfarin therapy. The strongest predictors of ischemic stroke and systemic thromboembolism are a history of stroke or a transient ischemic episode and mitral stenosis. When patients with AF and a previous ischemic stroke are treated with aspirin, the risk for another stroke is very high, in the range of 10% to 12% per year. At the other end of the risk spectrum are patients with lone AF, whose cumulative 15-year risk for stroke was reported to be in the range of 1% to 2%. Aside from previous stroke, the best-established risk factors for stroke in patients with nonvalvular AF are diabetes (relative risk, 1.7), hypertension (relative risk, 1.6), heart failure (relative risk, 1.4), and age 70 years or older (relative risk, 1.4 per decade).8 A simple clinical scheme to risk-stratify patients on the basis of major risk factors is the CHADS2 (cardiac failure, hypertension, age, diabetes, stroke) score. Each of the first four risk factors is worth 1 point, and a previous stroke or transient ischemic event is worth 2 points. There is a direct relationship between the CHADS2 score and the annual risk for stroke in the absence of aspirin or warfarin therapy. The clinical value of the CHADS2 score lies in its simplicity and predictive value. However, recent studies have demonstrated that the CHA2DS2-VASc score more accurately discriminates low-risk from intermediate-risk patients.9 In this risk score system, cardiac failure, hypertension, diabetes, vascular disease, 65 to 74 years of age, and female sex are worth 1 point each, whereas age 75 years or older and previous stroke or transient ischemic event are worth 2 points. The annual risk for stroke is zero or close to zero when the CHA2DS2-VASc score is 0, as opposed to approximately 2% when the CHADS2 score is 0.10 A score of 1 is associated with an annual stroke risk of approximately 3% with the CHADS2 score versus 0.7% with the CHA2DS2-VASc score (Fig. 38-6). A large-scale study demonstrated that renal failure is also an independent risk factor for stroke in patients with AF.11 The relative risk for a thromboembolic event in the absence of anticoagulation was 1.4 in patients with an estimated glomerular filtration rate lower than 45 mL/min/1.73 m2. The predictive strength of this degree of renal failure for a thromboembolic event appears to be equivalent to that of heart failure and advanced age. Therefore, it may be appropriate to take renal failure into account in evaluating the risk profile of a patient with AF. By definition, the burden of AF is greater in patients with persistent AF than in those with paroxysmal AF. It may seem reasonable to assume that the risk for stroke is lower in patients with occasional episodes of self-limited AF than in those with AF continuously. However, the data available in fact indicate that the risk for thromboembolic complications is the same in patients with paroxysmal and persistent AF. Even 15 minutes of AF may be long enough to result in local cardiac platelet activation and endothelial dysfunction, which predispose to thrombus formation during an acute episode of AF.12 Therefore, the type of AF should not be taken into account in risk-stratifying AF patients for thromboembolic risk. Modern-day dual-chamber pacemakers and implantable cardioverter-defibrillators (ICDs) are capable of detecting short episodes of asymptomatic AF that would otherwise not have been detected clinically. In a recent multicenter prospective study, subclinical atrial tachyarrhythmias (atrial rate >190 beats/min for >6 minutes) were detected by device interrogation in 10.1% of patients 65 years or older with hypertension and no history of AF who received a pacemaker or ICD.13 Subclinical atrial tachyarrhythmias were independently associated with a 2.5-fold increase in the risk for stroke. An important consideration in patients treated with an oral anticoagulant is the risk for bleeding. Several risk stratification scoring systems have been developed to assess a patient’s susceptibility to hemorrhagic complications. The scoring system with the best balance of simplicity and accuracy is the HAS-BLED score.14 The components of this score are hypertension, abnormal renal or liver function, stroke, bleeding history or predisposition, labile international normalized ratio (INR), older age (>75 years), and concomitant drug (antiplatelet agent or nonsteroidal anti-inflammatory drug) or alcohol use. Each of these components is worth 1 point. As the score increases from 0 to the maximum of 9, there is a stepwise increase in the risk for bleeding in patients treated with warfarin. For example, in one study the annual rate of major bleeding was 1.1% in patients with a HAS-BLED score of 0, 3.7% with a score of 3, and 12.5% with a score of 5.15 In two large-scale cohort studies totaling 132,372 patients16 and 170,292 patients17 with nonvalvular AF, the CHA2DS2-VASc and HAS-BLED scores were calculated for each patient. The net clinical benefit of warfarin was defined as the number of strokes while not taking warfarin minus the number of intracranial bleeding episodes while taking warfarin. In both studies, warfarin was associated with a net clinical benefit except when the CHA2DS2-VASc score was 0. In patients with a CHA2DS2-VASc score of 1 or higher, the risk for stroke in the absence of warfarin exceeded the number of bleeding complications during treatment with warfarin. The results of these large cohort studies notwithstanding, the decision to institute anticoagulation in a patient in clinical practice should be individualized. At times it may be appropriate to not initiate anticoagulation in a patient with a CHA2DS2-VASc score of 1 or higher. For example, the annual risk for stroke in a patient with a CHA2DS2-VASc score of 2 is approximately 2%, which usually justifies the use of warfarin. However, if that patient has a HAS-BLED score of 5 or higher, which is associated with an annual risk for major bleeding of 12% or higher, it would be imprudent to treat that patient with warfarin. It should be noted that the HAS-BLED score was developed and validated in patients in whom warfarin was used for anticoagulation. Except for labile INR, it is likely that the components of the HAS-BLED score also apply to patients in whom a direct thrombin inhibitor or factor Xa inhibitor is used for anticoagulation. However, the predictive value of the HAS-BLED score in patients treated with one of the newer antithrombotic agents has not yet been determined. Aspirin does not prevent thromboembolic complications as effectively as warfarin does in patients with AF. In a meta-analysis of five randomized clinical trials, aspirin reduced the risk for stroke by only 18%.7 In a recent large cohort study of patients with nonvalvular AF, aspirin had no therapeutic efficacy in preventing strokes.16 Therefore, if aspirin is used for prophylactic therapy, it should be used only in patients at lowest risk for thromboembolic complications (CHA2DS2-VASc score of 0). The 2011 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines still recommend aspirin for stroke prevention in patients with a CHADS2 score of 0 and either aspirin or an oral anticoagulant when the CHADS2 score is 1.18 Because of the negligible therapeutic effect of aspirin, a risk for bleeding complications that is close to the risk associated with oral anticoagulants, and the ability of the CHA2DS2-VASc score to accurately identify low-risk patients, the most recent guidelines of the European Society of Cardiology recommend no antithrombotic therapy when the CHA2DS2-VASc score is 0 and an individualized decision regarding no antithrombotic therapy versus an oral anticoagulant when the CHA2DS2-VASc score is 1.19 If aspirin is used for stroke prevention in patients with AF, the appropriate daily dose is 81 to 325 mg/day. No data are available to indicate superiority of a particular dose for prevention of thromboembolism. In patients with a CHADS2 score higher than 1 who are not able to tolerate anticoagulation with warfarin, combination therapy with aspirin and the platelet inhibitor clopidogrel is more efficacious than aspirin alone for prevention of thromboembolic complications. In a randomized, double-blind clinical trial (ACTIVE-A), all patients with AF and one or more risk factors for stroke who were not suitable candidates for anticoagulation with warfarin were treated with 75 to 100 mg/day of aspirin.20 The patients were randomly assigned to also receive either 75 mg/day of clopidogrel or a matching placebo. The primary outcome was a composite of stroke, myocardial infarction, systemic embolism, and vascular death. When compared with placebo, clopidogrel reduced the risk for stroke by 28% and risk for the primary outcome by 11% but increased the risk for major hemorrhage. The study demonstrated that for every 1000 patients treated with the combination of aspirin plus clopidogrel instead of aspirin alone, 28 strokes (17 fatal or disabling) and 6 myocardial infarctions would be prevented, at a cost of 20 major bleeding episodes (3 fatal). Therefore, in high-risk patients who are not suitable candidates for warfarin, the benefits of combination therapy with aspirin plus clopidogrel outweigh the risk. A meta-analysis of the major randomized clinical trials in which warfarin was compared with placebo for prevention of thromboembolism in patients with AF demonstrated that warfarin reduced the risk for all strokes (ischemic and hemorrhagic) by 61%.7 The target INR should be 2.0 to 3.0. This range of INRs provides the best balance between stroke prevention and hemorrhagic complications. In clinical practice, maintenance of the INR in the therapeutic range has been challenging, and a large proportion of patients often have an INR lower than 2.0. A large prospective study of community-based practices demonstrated that the mean time in the therapeutic range in patients treated with warfarin was just 66% and that the time in the therapeutic range was less than 60% in 34% of patients.21 Maintaining the INR at a level of 2.0 or higher is important because even a relatively small decrease in the INR from 2.0 to 1.7 more than doubles the risk for stroke. Furthermore, the data available indicate that the combination of aspirin and low-intensity anticoagulation with warfarin is inferior to warfarin in the standard therapeutic range for stroke prevention. The annual risk for a major hemorrhagic complication during anticoagulation with warfarin is in the range of 1% to 2%, and a strong predictor of major bleeding events is an INR higher than 3.0. For example, the risk for intracranial bleeding is approximately twice as high at an INR of 4.0 than at 3.0. This emphasizes the importance of maintaining the INR in the range of 2.0 to 3.0. Some studies have indicated that advanced age can be a risk factor for intracranial hemorrhage in patients with AF who are treated with warfarin. The fear of hemorrhagic complications may lead some clinicians to favor the use of aspirin over warfarin in older adults. However, recent data indicate that the risk-to-benefit ratio of warfarin is more favorable than that of aspirin, even in patients older than 75 years. A randomized clinical trial (the Birmingham Atrial Fibrillation Treatment of the Aged Study) enrolled 973 patients older than 75 years (mean age, 82 years) with AF and randomly assigned them to treatment with 75 mg/day of aspirin or with warfarin adjusted to maintain an INR of 2.0 to 3.0.22 The primary endpoint was the composite of stroke (ischemic or hemorrhagic), intracranial hemorrhage, and arterial embolism, and the mean duration of follow-up was 2.7 years. The annual risk for the composite endpoint was significantly higher in the aspirin group (3.8%) than in the warfarin group (1.8%), even when the analysis was limited to patients older than 85 years. These data suggest that age should not be considered a contraindication to treatment with warfarin in patients with AF. It is well established that genetic factors influence the dose of warfarin required to maintain the INR within the therapeutic range. Several single nucleotide polymorphisms that affect warfarin metabolism have been identified. Algorithms based on pharmacogenetic (see Chapter 9) and clinical factors improve the accuracy of warfarin dose initiation more than do algorithms based only on clinical factors, particularly for outliers who require 21 mg/wk or less or 49 mg/wk or more of warfarin to maintain a therapeutic INR.23 However, a randomized study demonstrated that warfarin dosing that took into account the results of genotyping for CYP2C9 (a cytochrome P-450 isoform) and VKORC1 (a vitamin K epoxide reductase complex subunit) did not improve the time in the therapeutic range, which was approximately 70% in both groups.24 Additional studies are required to determine whether the clinical benefits of genotyping of warfarin candidates justify the cost of genetic testing. Direct thrombin inhibitors and factor Xa inhibitors have several advantages over vitamin K antagonists such as warfarin, the most notable being a fixed dosing regimen, which eliminates the need for monitoring of a laboratory test such as the INR. Dabigatran, an oral direct thrombin inhibitor, and rivaroxaban, a factor Xa inhibitor, were approved by the Food and Drug Administration for prevention of stroke/embolism in patients with nonvalvular AF in 2010 and 2011, respectively. Another factor Xa inhibitor, apixaban, was expected to gain Food and Drug Administration approval in 2013. Randomized clinical trials have demonstrated that each of these three new oral anticoagulants is noninferior or superior to warfarin in efficacy and safety. These studies included patients with nonvalvular AF who had risk factors for stroke. In the RE-LY study, dabigatran at a dose of 150 mg twice daily was associated with a lower risk for stroke and systemic embolism than warfarin was and a similar rate of major hemorrhage.25 In the ROCKET-AF study, rivaroxaban at a dose of 20 mg once daily was noninferior to warfarin for prevention of stroke/systemic embolism and was associated with a risk for major bleeding that did not differ from that of warfarin.26 However, intracranial hemorrhage and fatal bleeding were less common with rivaroxaban. In the ARISTOTLE study, apixaban at a dose of 5 mg twice daily was superior to warfarin in prevention of stroke/systemic embolism and was associated with a lower risk for hemorrhagic complications and lower mortality (Fig. 38-7).27 The newer oral anticoagulants, in addition to eliminating the need for laboratory monitoring, have other advantages over warfarin: fewer drug interactions, no food interactions, and a rapid onset of action that obviates the need for bridging therapy. However, they also have some disadvantages in comparison to warfarin: higher cost, more gastrointestinal side effects in the case of dabigatran, twice-daily dosing in the case of dabigatran and apixaban, and absence of a laboratory test to verify compliance. Furthermore, these agents cannot be used safely in patients with severe renal disease. Another limitation is that the effects of the newer anticoagulants may be difficult to reverse in patients with an overdose or hemorrhage. For example, in a 2011 study, a single bolus of 50 IU/kg of prothrombin complex concentrate was demonstrated to quickly and completely reverse the anticoagulant effect of rivaroxaban, but not dabigatran.28 Nonetheless, for many patients with AF, the advantages of the newer anticoagulants outweigh the disadvantages. The major professional societies have incorporated recommendations regarding the use of factor Xa and/or direct thrombin inhibitors into their most recent updates of guidelines for the management of AF. The practice guidelines of the American College of Cardiology/American Heart Association/Heart Rhythm Society recommend dabigatran as a useful alternative to warfarin for prevention of stroke/systemic embolism in patients with nonvalvular paroxysmal or persistent AF and risk factors for stroke. However, this recommendation is limited to patients without a prosthetic valve, with creatinine clearance lower than 15 mL/min, or with impaired clotting function as a result of advanced liver disease.29 The European Society of Cardiology guidelines recommend dabigatran, rivaroxaban, or apixaban for patients with AF in whom maintenance of a therapeutic INR during treatment with warfarin is difficult and state that one of these newer anticoagulants should be considered instead of dose-adjusted warfarin for most patients with nonvalvular AF, based on their net clinical benefits.19 The guidelines also recommend that these agents not be used in patients with a creatinine clearance lower than 30 mL/min. An issue that has not been addressed in a randomized clinical trial is whether the newer oral anticoagulants provide adequate protection from the thromboembolic complications of transthoracic cardioversion. Although not studied prospectively, the safety of dabigatran in patients undergoing cardioversion was evaluated in a post hoc analysis of the RE-LY study.30 A subset of 1336 patients underwent cardioversion after 3 weeks or more of treatment with dabigatran, 150 mg twice daily, or dose-adjusted warfarin with an INR of 2.0 to 3.0. The stroke/systemic embolism rate at 30 days did not differ significantly between the dabigatran group (0.3%) and the warfarin group (0.6%). There was also no difference between the two groups in the rate of major bleeding (0.6% in both groups). These data suggest that dabigatran is a safe and effective alternative to warfarin in patients requiring cardioversion. However, because patient compliance and a therapeutic effect of dabigatran cannot be confirmed by laboratory testing, a precardioversion transesophageal echocardiogram to rule out a left atrial thrombus may be appropriate more often in patients treated with dabigatran than in those treated with warfarin. The onset of action of dabigatran, rivaroxaban, and apixaban is approximately 1.5 to 2 hours after a dose. The half-lives of dabigatran and apixaban range between 10 and 16 hours, and the half-life of rivaroxaban is 5 to 9 hours. These anticoagulants lose most of their effect by 24 hours after discontinuation. The rapid onset of action and washout eliminate the need for bridging therapy with heparin when treatment with one of the new anticoagulants is interrupted for a surgical or invasive procedure. In a recent study of patients treated with dabigatran who underwent radiofrequency catheter ablation of AF, dabigatran was withheld on the morning of the procedure.31 The study included a comparison group of patients who underwent radiofrequency catheter ablation of AF during uninterrupted therapy with warfarin at an INR of 2.0 to 3.0. Major hemorrhagic complications occurred significantly more often in the dabigatran group (6%) than in the warfarin group (1%). The results of this study demonstrate that dabigatran should be withheld for at least 24 hours before an invasive or surgical procedure. Low-molecular-weight heparin has a longer half-life than unfractionated heparin does and a predictable antithrombotic effect that is attained with a fixed dosage administered subcutaneously twice a day. Because low-molecular-weight heparin can be self-injected by patients outside the hospital, it is a practical alternative to unfractionated heparin for initiation of anticoagulation with warfarin in patients with AF. Bridging therapy with low-molecular heparin should be continued until the INR is 2.0 or higher. Because of its high cost, low-molecular-weight heparin is rarely used in clinical practice as a substitute for long-term conventional anticoagulation. Low-molecular-weight heparin is typically used as a temporary bridge to therapeutic anticoagulation when therapy with warfarin is initiated or in high-risk patients for a few days before and after a medical or dental procedure when anticoagulation with warfarin has been suspended. Approximately 90% of left atrial thrombi form in the appendage, and therefore successful excision or closure of the left atrial appendage should markedly reduce the risk for thromboembolic complications in patients with AF. Surgical techniques consist of either excision or closure by suturing or stapling. The efficacy of these techniques is variable and probably dependent on both technique and the operator. Postoperative transesophageal echocardiography demonstrated that the appendage was closed successfully in only 40% of 137 patients.32 The rate of successful closure was higher when the appendage was excised (73%) than when it was closed by suturing (23%). Of note is that complete closure was never achieved by stapling of the appendage. Left atrial appendage thrombi were never seen on transesophageal echocardiography after excision of the appendage but were observed in 41% of patients who underwent closure of the appendage. Therefore, transesophageal echocardiography should be performed after surgical closure of the left atrial appendage to confirm successful closure before discontinuation of anticoagulation. Closure of the left atrial appendage can also be achieved percutaneously with an implanted device intended to seal the appendage. A randomized clinical trial (PROTECT AF) compared the efficacy of a percutaneous closure device versus warfarin for prevention of thromboembolic complications in 707 patients with AF and a CHADS2 score of 1 or higher.33 The device was found to be noninferior to warfarin for the primary efficacy composite endpoint of stroke, systemic emboli, and cardiovascular death and superior to warfarin for hemorrhagic stroke (91% reduction). The complication rate was approximately four times higher in the device arm, with the most common complication being pericardial effusion. The study demonstrated that percutaneous closure of the left atrial appendage is an effective alternative to warfarin in patients with AF. Another recent study showed that the risk for pericardial effusion declines significantly as operator experience increases.34 Approval of the left atrial appendage closure device by the Food and Drug Administration awaits additional safety data. It is likely that the left atrial appendage closure device will have its greatest utility in high-risk patients with AF who cannot tolerate or refuse to take an oral anticoagulant. Patients who go to the emergency department because of AF generally have a rapid ventricular rate, and control of the ventricular rate is most rapidly achieved with intravenous diltiazem or esmolol. If the patient is hemodynamically unstable, immediate transthoracic cardioversion may be appropriate. If the AF has been present for more than 48 hours or if the duration is unclear and the patient is not already receiving an anticoagulant, cardioversion should be preceded by transesophageal echocardiography to rule out a left atrial thrombus. If the patient is hemodynamically stable, the decision to restore sinus rhythm by cardioversion is based on several factors, including symptoms, previous AF episodes, age, left atrial size, and current antiarrhythmic drug therapy. For example, in an elderly patient whose symptoms resolve once the ventricular rate is controlled and who has already had early recurrences of AF despite rhythm-control drug therapy, further attempts at cardioversion are not usually appropriate. On the other hand, cardioversion is generally appropriate for patients with symptomatic AF who are seen with a first episode of AF or who have had long intervals of sinus rhythm between previous episodes. If cardioversion is decided on for a hemodynamically stable patient with AF that does not appear to be self-limited, two management decisions must be made: early versus delayed cardioversion and pharmacologic versus electrical cardioversion. The advantages of early cardioversion are rapid relief of symptoms, avoidance of the need for transesophageal echocardiography or therapeutic anticoagulation for 3 to 4 weeks before cardioversion if it is performed within 48 hours of the onset of AF, and possibly a lower risk for early recurrence of AF because of less atrial remodeling (see Chapter 33). A reason to defer cardioversion is the unavailability of transesophageal echocardiography in an unanticoagulated patient with AF of unclear duration or a duration longer than 48 hours. Other reasons include a left atrial thrombus noted on transesophageal echocardiography (see Fig. 15-91), a suspicion (based on previous AF episodes) that AF will convert spontaneously within a few days, or a correctable cause of AF (e.g., hyperthyroidism). When cardioversion is performed early in the course of an episode of AF, either pharmacologic or electrical cardioversion is an option. Pharmacologic cardioversion has the advantage of not requiring general anesthesia or deep sedation. In addition, the probability of an immediate recurrence of AF may be lower with pharmacologic cardioversion than with electrical cardioversion. However, pharmacologic cardioversion is associated with a risk for adverse drug effects and is not as effective as electrical cardioversion. Pharmacologic cardioversion is very unlikely to be effective if the duration of AF is longer than 7 days. Drugs that can be administered intravenously for cardioversion of AF consist of ibutilide, procainamide, and amiodarone. For AF episodes shorter than 2 to 3 days in duration, the efficacy of these drugs is approximately 60% to 70% for ibutilide, 40% to 50% for amiodarone, and 30% to 40% for procainamide. To minimize the risk for QT prolongation and polymorphic ventricular tachycardia (torsades de pointes; see Chapter 37), use of ibutilide should be limited to patients with an ejection fraction higher than 35%. Acute pharmacologic cardioversion of AF can also be attempted with orally administered drugs in patients without structural heart disease. The most commonly used oral agents for acute conversion of AF are propafenone (300 to 600 mg) and flecainide (100 to 200 mg). It is prudent to administer these drugs under surveillance the first time that they are used. If no adverse drug effects are observed, the patient may then be an appropriate candidate for episodic, self-administered antiarrhythmic drug therapy on an outpatient basis. The efficacy of transthoracic cardioversion is approximately 95%. Biphasic waveform shocks convert AF more effectively than do monophasic waveform shocks and allow the use of lower energy shocks, which result in a lower risk for skin irritation. An appropriate first-shock strength using a biphasic waveform is 150 to 200 J followed by higher output shocks if needed. If a 360-J biphasic shock is unsuccessful, ibutilide should be infused before another shock is delivered because it lowers the defibrillation energy requirement and improves the success rate of transthoracic cardioversion. Two types of failure of transthoracic cardioversion occur in patients with AF. The first type is complete failure to restore sinus rhythm. In this situation, an increase in shock strength or infusion of ibutilide often results in successful cardioversion. The second type of failure is immediate recurrence of AF within a few seconds of successful conversion to sinus rhythm. The incidence of immediate recurrence of AF is approximately 25% for episodes shorter than 24 hours in duration and approximately 10% for episodes longer than 24 hours in duration. For this type of failure of cardioversion, an increase in shock strength is of no value. If the patient has not been receiving an oral rhythm-control agent, infusion of ibutilide may be helpful for prevention of an immediate recurrence of AF. Regardless of whether cardioversion is performed pharmacologically or electrically, therapeutic anticoagulation is necessary for 3 weeks or longer before cardioversion to prevent thromboembolic complications if the AF has been ongoing for more than 48 hours. If the time of onset of AF is unclear, for the sake of safety, the duration of AF should be assumed to be greater than 48 hours. These patients should receive therapeutic anticoagulation for 4 weeks after cardioversion to prevent the thromboembolic complications that may occur because of atrial stunning. If the duration of AF is known to be less than 48 hours, cardioversion can be performed without anticoagulation. To improve the safety margin, it may be appropriate to use a 24-hour cutoff for the AF duration, which allows safe cardioversion without anticoagulation. When the duration of AF is longer than 48 hours or unclear, an alternative to 3 weeks of therapeutic anticoagulation before cardioversion is anticoagulation with heparin and transesophageal echocardiography to check for a left atrial thrombus. If no thrombi are seen, the patient can safely be cardioverted but still requires 4 weeks of therapeutic anticoagulation after cardioversion to prevent thromboembolism related to atrial stunning. The major clinical benefit of the transesophageal echocardiography–guided approach over the conventional approach is that sinus rhythm is restored several weeks sooner. When compared with the conventional approach, the transesophageal echocardiography–guided approach has not been found to reduce the risk for stroke or major bleeding or to affect the proportion of patients still in sinus rhythm at 8 weeks after cardioversion. Several randomized studies have compared a rate-control strategy with a rhythm-control strategy in patients with AF. The largest study by far was the AFFIRM study, which consisted of 4060 patients with a mean age of 70 years who had AF for 6 hours to 6 months.35 At 5 years of follow-up, the prevalence of sinus rhythm was 35% in the rate-control arm and 63% in the rhythm-control arm. No significant difference was noted between the two study arms in total mortality, stroke rate, or quality of life. The percentage of patients requiring hospitalization was significantly lower in the rate-control arm (73%) than in the rhythm-control arm (80%), and the incidence of adverse drug effects such as torsades de pointes was also significantly lower in the rate-control arm (0.2% versus 0.8%). The authors of the AFFIRM study concluded that there is no survival advantage of a rhythm-control strategy over a rate-control strategy and that a rate-control strategy has advantages such as a lower probability of hospitalization and adverse drug effects. In a post hoc analysis of the AFFIRM study, the relationship between sinus rhythm, treatment, and survival was determined by an on-treatment analysis instead of the intention-to-treat analysis used in the original report.36 Sinus rhythm was found to be independently associated with lower mortality (hazard ratio, 0.53), and antiarrhythmic drug therapy was independently associated with increased mortality (hazard ratio, 1.49). Therefore, the potential benefit of maintaining sinus rhythm with antiarrhythmic drugs was negated by the adverse effects of the antiarrhythmic drug therapy. This suggested that therapies that maintain sinus rhythm without major adverse effects may have a beneficial effect on survival. The results of the AFFIRM study should not be applied routinely to all patients with AF. The decision to pursue a rhythm-control strategy versus a rate-control strategy should be individualized, with several factors being taken into account, including the nature, frequency, and severity of symptoms; the length of time that AF has been present continuously in patients with persistent AF; left atrial size; comorbid conditions; the response to previous cardioversions; age; the side effects and efficacy of the antiarrhythmic drugs already used to treat the patient; and the patient’s preference.
Atrial Fibrillation
Clinical Features, Mechanisms, and Management
Electrocardiographic Features
Classification of Atrial Fibrillation
Epidemiology of Atrial Fibrillation
Mechanisms of Atrial Fibrillation
Genetic Factors
Causes of Atrial Fibrillation
Clinical Features
Diagnostic Evaluation
Prevention of Thromboembolic Complications
Risk Stratification
Aspirin and Other Antithrombotic Agents
Warfarin
Newer Oral Anticoagulants
Low-Molecular-Weight Heparin
Excision or Closure of the Left Atrial Appendage
Acute Management of Atrial Fibrillation
Long-Term Management of Atrial Fibrillation
Pharmacologic Rate Control Versus Rhythm Control
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Atrial Fibrillation
38