Atrial arrhythmias (AAs) are a common complication in adult patients with congenital heart disease. We sought to compare the lifetime prevalence of AAs in patients with right- versus left-sided congenital cardiac lesions and their effect on the prognosis. A congenital heart disease diagnosis was assigned using the International Disease Classification , Ninth Revision , diagnostic codes in the administrative databases of Quebec, from 1983 to 2005. Patients with AAs were those diagnosed with an International Disease Classification , Ninth Revision , code for atrial fibrillation or intra-atrial reentry tachycardia. To ensure that the diagnosis of AA was new, a washout period of 5 years after entry into the database was used, a period during which the patient could not have received an International Disease Classification , Ninth Revision , code for AA. The cumulative lifetime risk of AA was estimated using the Practical Incidence Estimators method. The hazard ratios (HRs) for mortality, morbidity, and cardiac interventions were compared between those with right- and left-sided lesions after adjustment for age, gender, disease severity, and cardiac risk factors. In a population of 71,467 patients, 7,756 adults developed AAs (isolated right-sided, 2,229; isolated left-sided, 1,725). The lifetime risk of developing AAs was significantly greater in patients with right- sided than in patients with left-sided lesions (61.0% vs 55.4%, p <0.001). The HR for mortality and the development of stroke or heart failure was similar in both groups (HR 0.96, 95% confidence interval [CI] 0.86 to 1.09; HR 0.94, 95% CI 0.80 to 1.09; and HR 1.10, 95% CI 0.98 to 1.23, respectively). However, the rates of cardiac catheterization (HR 0.63, 95% CI 0.55 to 0.72), cardiac surgery (HR 0.40, 95% CI 0.36 to 0.45), and arrhythmia surgery (HR 0.77, 95% CI 0.6 to 0.98) were significantly less for patients with right-sided lesions. In conclusion, patients with right-sided lesions had a greater lifetime burden of AAs. However, their morbidity and mortality were no less than those with left-sided lesions, although the rate of intervention was substantially different.
Experimental studies in rabbits have suggested that for a similar pressure increase, the right atrium is more susceptible to the development of atrial fibrillation than the left atrium. From the these findings, we hypothesized that at a population level patients with congenital heart disease (CHD) and right-sided heart lesions and, hence, increased right atrial pressure, would have a greater lifetime risk of developing atrial arrhythmias (AAs) than would patients with left-sided heart lesions. We also sought to determine whether the presence of AAs affected patients’ mortality and morbidity differently according to whether they had right-sided versus left-sided congenital cardiac lesions.
Methods
In Quebec, Canada’s second largest province, a unique healthcare number is assigned to all persons at birth and is systematically linked to all diagnoses, hospitalizations, and health services rendered for the duration of a person’s life. The administrative databases include the physician’s services and drug claims database (Régie de l’assurance-maladie du Québec), the hospital discharge summary database (Med-Echo), and the Quebec Health Insurance Board.
A province-wide, population-based CHD database was created at our institution by merging the province’s 3 administration databases. During the study period, the diagnostic codes adhered to the International Disease Classification , Ninth Revision (ICD-9). Patients were identified with CHD if they had at least one diagnostic code for CHD and/or had undergone a CHD-specific surgical procedure. Provider codes were used to select the diagnoses made by the primary care physicians or cardiovascular medical specialists and procedures performed by cardiovascular surgeons. Patients were assigned 1 or 2 CHD diagnoses using a previously defined hierarchical algorithm. All information was cross-referenced between the outpatient and inpatient data sources. By law, the attestation of death is sent to the Quebec Health Insurance Board, making documentation of death complete in the database, whether it occurred in or out of the hospital. The CHD database in the province of Quebec therefore contained comprehensive longitudinal, demographic, diagnostic, and therapeutic records of all patient-linked encounters with the healthcare system from January 1, 1983 to December 31, 2005 (inclusive) for all Quebec residents identified with CHD.
The McGill University Health Centre ethics board and the Quebec government agency responsible for privacy of access to information approved the study.
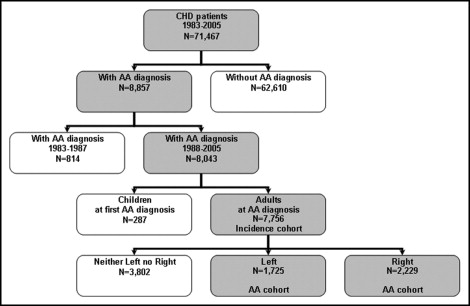
The study’s population cohorts were derived from the Quebec’s CHD database ( Figure 1 ). All patients were either adults or reached 18 years of age during the study period. The patients were included with AAs if a diagnosis of atrial fibrillation or intra-atrial reentry-tachycardia (ICD-9 code 4273) was made by selected specialists (anesthetists, cardiologists, cardiothoracic surgeons, emergency doctors, general practitioners, internists, neurologists, and pediatricians) during the 18-year study period. The adult CHD cohort with AAs was divided into 2 groups, those with right- and left-sided lesions, according to the ICD-9 diagnoses and formed the study population. The details of the right- and left-sided lesions included in the present study are summarized in Table 1 . The CHD diagnoses that were associated with abnormal loading conditions on both sides of the heart (e.g., atrioventricular septal defects, transposition of the great vessels after arterial switch, and anomaly of the heart not otherwise specified), were excluded.
| Right-Sided Lesions | Left-Sided Lesions |
|---|---|
| Atrial septal defect | Bicuspid aortic valve |
| Ebstein’s anomaly | Aortic coarctation |
| Single ventricle physiology (Fontan) | Ventricular septal defect |
| Tetralogy of Fallot | Patent ductus arteriosus |
| Pulmonary stenosis | Mitral valve disease |
| Tricuspid valve disease | Aortic valve disease |
For an estimation of the age-related lifetime risk, we defined an incidence cohort that included subjects free of AA on January 1, 1988 who were or became adults from 1988 to 2005 ( Figure 1 ). The incidence cohort started in 1988 (ie, 5 years after the start of the database) to allow the identification and exclusion of prevalent AA cases from the incidence cohort. Those subjects in the incidence cohort who developed AA were considered incident AA cases and were used in the calculation of the age-specific lifetime AA risk.
We defined an AA cohort by considering patients at the first AA diagnosis in their lifetime and followed them until the first event or the end of the study, whichever came first.
Outcomes were defined as mortality, morbidity, and interventions, and the combination of the 3 (any adverse event). Mortality was defined as death during the follow-up period after the first AA diagnosis. Morbidity was defined as an episode of congestive heart failure and/or stroke during the follow-up period after the first AA diagnosis and was measured using the ICD-9 diagnostic codes in the 2 administrative databases. Interventions were separated into surgery and percutaneous interventions and were identified using the procedural billing codes in the medical claims database. An adverse event was defined as the first occurrence of any of the 3 clinical outcomes during the follow-up period.
Confounders were defined as clinical diagnoses known to be risk factors for AA and each of the 3 outcomes. These included a history of hypertension, coronary artery disease, diabetes, stroke, heart failure, and recent cardiac surgery. The medical confounders were measured in the 5 years before time 0 using the ICD-9 codes in the 2 administrative databases, and recent cardiac surgery was measured in the 30 days before time 0 using the procedure claim codes.
Descriptive statistics included medians, interquartile range, and proportions. The lifetime cumulative incidence of AA in patients with adult CHD with right- versus left-sided lesions was calculated in the incidence cohort using the Practical Incidence Estimators method. From these analyses, we report the lifetime risk of developing AA and the corresponding 95% confidence intervals (CIs).
Cox multiple regression analysis was performed in the AA cohort using the time of the first AA diagnosis as time 0 and adjusting for gender, age, hypertension, diabetes, ischemic heart disease, stroke, and heart failure, one model for each adverse outcome. All confounders adjusted for through modeling were defined a priori and kept in the model, regardless of their statistical significance. The proportional hazards assumption was tested using the −log (log[S(T)]) plot, and no violation was detected. From this analysis, we report the hazard ratios (HRs) for left- versus right-sided lesions and 95% CIs. All statistical analyses were performed using the Statistical Analysis Systems statistical software, version 9.1 (SAS Institute, Cary, North Carolina).
Results
In a CHD population of 71,467 patients, 8,043 (11%) had a diagnosis of AAs during the study period. Most of these patients (7,756) were adults. Of those, 2,229 patients had a diagnosis of isolated right-sided lesions and 1,725 patients a diagnosis of isolated left-sided lesions (3,802 patients were excluded because of a cardiac diagnosis involving both the right and left side of the heart or because of an unspecified cardiac diagnosis; Figure 1 ). The baseline characteristics of the study population are listed in Table 2 . Patients with right-sided disease were younger, had a female predominance, and had fewer cardiac co-morbidities.
| Variable | 2005 Prevalence Cohort | ||
|---|---|---|---|
| AA, Right (n = 2,229) | AA, Left (n = 1,725) | p Value | |
| Median age in 2005 (interquartile range) | 63 (49–75) | 68 (54–77) | <0.0001 |
| Men | 922 (41%) | 927 (54%) | <0.0001 |
| Cardiac diagnosis | |||
| Tetralogy of Fallot | 180 (8%) | — | — |
| Transposition of great arteries | 115 (5%) | — | — |
| Truncus arteriosus | 36 (2%) | — | — |
| Ebstein’s anomaly | 39 (2%) | — | — |
| Atrial septal defect | 1,622 (73%) | — | — |
| Anomalous pulmonary artery | 38 (2%) | — | — |
| Anomalous pulmonary vein | 145 (6%) | — | — |
| Congenital tricuspid valve defect | 46 (2%) | — | — |
| Ventricular septal defect | — | 398 (23%) | — |
| Patent ductus arteriosus | — | 58 (3%) | — |
| Coarctation of the aorta | — | 62 (3%) | — |
| Congenital aortic stenosis | — | 447 (26%) | — |
| Congenital aortic insufficiency | — | 203 (12%) | — |
| Congenital mitral stenosis | — | 20 (1%) | — |
| Congenital mitral valve insufficiency | — | 537 (31%) | — |
| Co-morbidity | |||
| Heart failure | 531 (24%) | 550 (32%) | <0.0001 |
| Cardiac surgery | 80 (4%) | 165 (10%) | <0.0001 |
| Acute myocardial infarction | 66 (3%) | 114 (7%) | <0.0001 |
| Diabetes | 258 (12%) | 241 (14%) | 0.02 |
| Hypertension | 766 (34%) | 726 (43%) | <0.0001 |
| Stroke | 198 (9%) | 149 (9%) | 0.78 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


