Atrial Arrhythmias
Mechanisms of arrhythmias
Under certain circumstances cardiac cells in any part of the heart may take on the role of pacemaker of the heart. Such a pacemaker is called an ectopic pacemaker (a pacemaker other than the sinus node). The result can be ectopic beats or rhythms. These rhythms are identified according to the location of the ectopic pacemaker (for example, atrial, junctional, or ventricular). The three basic mechanisms that are responsible for ectopic beats and rhythms are altered automaticity, triggered activity, and reentry:
▪ Altered automaticity — Normally the automaticity of the sinus node exceeds that of all other parts of the conduction system, allowing it to control the heart rate and rhythm. Pacemaker cells in other areas of the heart also have the property of automaticity, including cells in the atria, atrioventricular (AV) junction, and the ventricles. The rates of these other pacemaker sites are slower. Therefore, they’re suppressed by the sinus node under normal circumstances. Because the inherent firing rate of the pacemaker cells of the sinus node is faster than the other pacemaker sites, it is the dominant and primary pacemaker of the heart. An ectopic pacemaker site can take over the role of pacemaker either because it usurps control from the sinus node by accelerating its own automaticity (enhanced automaticity) or because the sinus node relinquishes its role by decreasing its automaticity. Conditions that may predispose cardiac cells to altered automaticity include myocardial ischemia or injury, hypoxia, an increase in sympathetic tone, digitalis toxicity, hypokalemia, and hypocalcemia.
▪ Triggered activity — Triggered activity results from abnormal electrical impulses that occur during repolarization when cells are normally quiet. The ectopic pacemaker cells may depolarize more than once after stimulation by a single electrical impulse. Triggered activity may result in atrial, junctional, or ventricular beats occurring singly, in pairs, in runs (3 or more beats), or as a sustained ectopic rhythm. Causes of triggered activity may include myocardial ischemia or injury, hypoxia, an increase in sympathetic tone, and digitalis toxicity.
▪ Reentry — Normally an impulse spreads through the heart only once. With reentry, an impulse can travel through an area of myocardium, depolarize it, and then reenter that same area to depolarize it again. Reentry involves a circular movement of the impulse, which continues as long as it encounters receptive cells. Reentry (like triggered activity) may result in atrial, junctional, or ventricular beats occurring singly, in pairs, in runs, or as a sustained ectopic rhythm. Common causes of reentry include myocardial ischemia or injury, hyperkalemia, and the presence of an accessory conduction pathway between the atria and the ventricles.
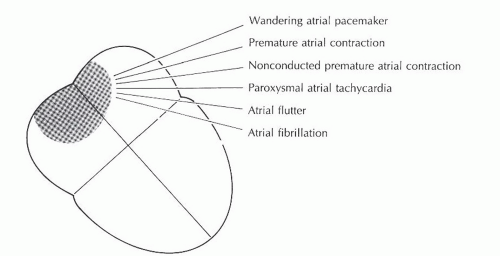
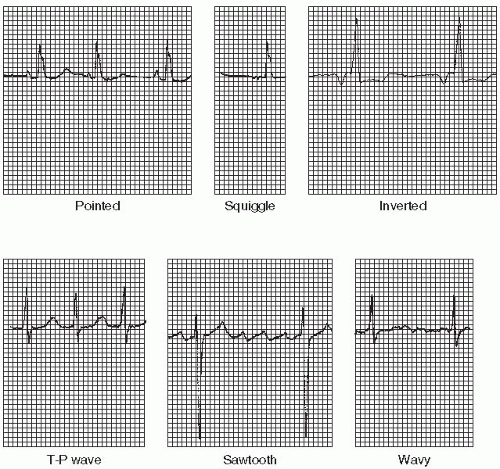
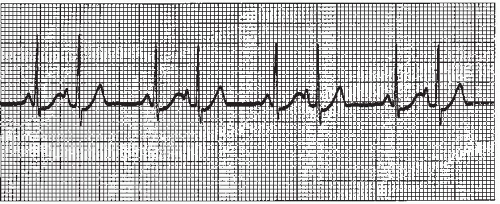
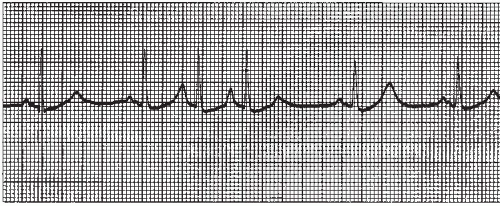
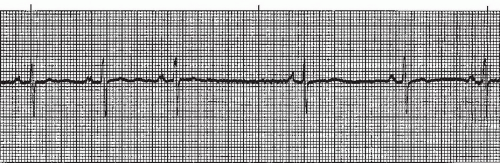
Atrial arrhythmias (Figure 7-1) originate from ectopic sites in the atria. Ectopic P waves from the atrium differ in morphology (shape) from the normal sinus P waves (Figure 7-2). For example, in slower atrial rhythms (premature atrial contractions, wandering atrial pacemaker) the P wave may appear as a small, pointed, and upright waveform; a small squiggle that is barely visible; or it may be inverted if the impulse originates from a site in the lower atrium near the AV junction. In faster atrial rhythms, the ectopic P wave is either superimposed on the preceding T wave, appears in a sawtooth pattern (atrial flutter), or is seen as a wavy baseline (atrial fibrillation).
Some atrial arrhythmias may be associated with rapid ventricular rates. Increases in heart rate decrease the length of time spent in diastole. If diastole is shortened, there is less time for coronary artery perfusion and less time for adequate ventricular filling. Thus, an excessively rapid heart rate may lead to myocardial ischemia and may compromise cardiac output.
Wandering atrial pacemaker
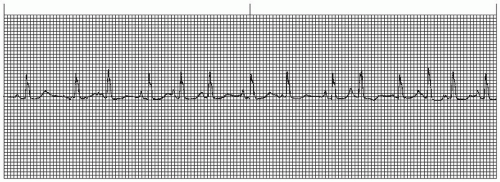
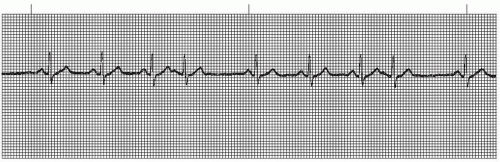
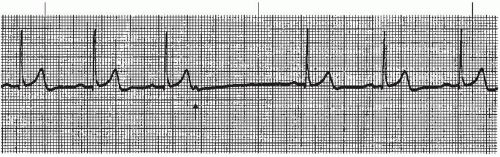
A wandering atrial pacemaker (WAP) (Figure 7-3 and Box 7-1) occurs when the pacemaker site shifts back and
forth between the sinus node and ectopic atrial sites. The P wave morphology will vary across the rhythm strip as the pacemaker “wanders” between the multiple sites. The ectopic P wave may appear as a small, pointed, and upright waveform; a small squiggle that is barely visible; or it may be inverted if the impulse originates from a site in the lower atrium near the AV junction. Generally, at least three different P-wave morphologies should be identified before making the diagnosis of WAP.
forth between the sinus node and ectopic atrial sites. The P wave morphology will vary across the rhythm strip as the pacemaker “wanders” between the multiple sites. The ectopic P wave may appear as a small, pointed, and upright waveform; a small squiggle that is barely visible; or it may be inverted if the impulse originates from a site in the lower atrium near the AV junction. Generally, at least three different P-wave morphologies should be identified before making the diagnosis of WAP.
Box 7-1. Wandering atrial pacemaker: Identifying ECG features
Rhythm: Regular or irregular
Rate: Usually normal (60 to 100 beats/minute) but may be slow (< than 60 beats/minute)
P waves: Vary in size, shape, and direction across rhythm strip; one P wave precedes each QRS complex
PR interval: Usually normal duration, but may be abnormal depending on changing pacemaker location
QRS complex: Normal (0.10 second or less)
The heart rate is usually normal, but may be slow. The rhythm may be regular or irregular (each impulse travels through the atria via a slightly different route). The PR interval is usually normal, but may be abnormal because of the different sites of impulse formation. The
QRS complex is normal in duration. The distinguishing feature of this rhythm is the changing P-wave morphology across the rhythm strip.
QRS complex is normal in duration. The distinguishing feature of this rhythm is the changing P-wave morphology across the rhythm strip.
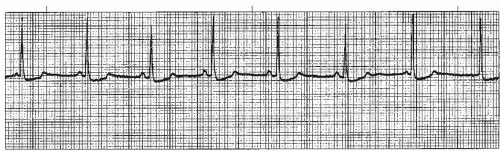 Figure 7-3. Wandering atrial pacemaker. Rhythm: Irregular Rate: 60 beats/minute P waves: Vary in size, shape, across rhythm strip PR interval: 0.10 to 0.14 second QRS complex: 0.04 to 0.08 second. |
WAP may be a normal phenomenon seen as a result of increased vagal effect on the sinoatrial (SA) node, slowing the sinus rate and allowing other pacemaker sites an opportunity to compete for control of the heart rate. It can also occur due to enhanced automaticity of atrial pacemaker cells that usurp pacemaker control from the SA node. WAP is commonly seen in patients with chronic obstructive pulmonary disease.
WAP usually isn’t clinically significant, and treatment is not indicated. If the heart rate is slow, medications should be reviewed and discontinued if possible. If the heart rate is slow and the patient is symptomatic, treatment of the rhythm is the same as for symptomatic sinus bradycardia.
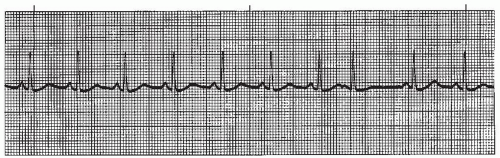
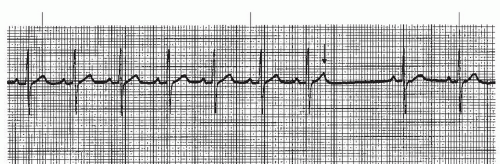
When WAP is associated with a heart rate greater than 100 beats per minute, the rhythm is called multifocal atrial tachycardia (MAT) (Figure 7-4). MAT is a relatively infrequent arrhythmia and is most commonly observed in patients with severe chronic obstructive pulmonary disease.
Premature atrial contraction
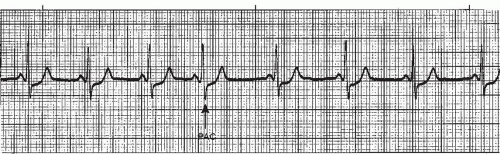
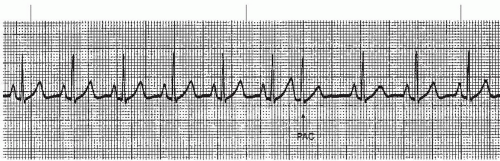
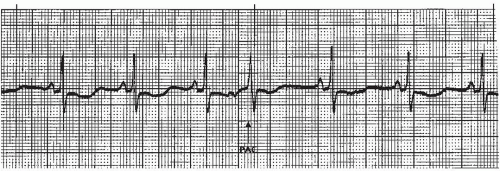
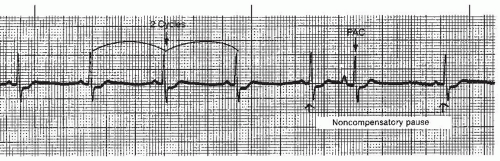
A premature atrial contraction (PAC) (Figures 7-5, 7-6, 7-7, 7-8, 7-9, 7-10, 7-11 and 7-12 and Box 7-2) is an early beat originating from an ectopic site in the atrium, which interrupts the regularity of the basic rhythm (usually a sinus rhythm). The premature beat occurs in addition to the basic underlying rhythm. PACs may originate from a single ectopic pacemaker site or from multiple sites in the atria. The early beat is characterized by a premature, abnormal P wave and a premature QRS complex that’s identical or similar to the QRS complex of the normally conducted beats, and is followed by a pause.
Box 7-2. Premature atrial contraction (PAC): Identifying ECG features
Rhythm: Underlying rhythm usually regular; irregular with PACs
Rate: That of underlying rhythm
P waves: P wave associated with PAC is premature and abnormal in size, shape, and direction (commonly appears small, upright, and pointed; may be inverted); abnormal P wave commonly found hidden in preceding T wave, distorting the T-wave contour
PR interval: Usually normal; not measurable if hidden in T wave
QRS complex: Premature; normal duration (0.10 second or less)
P-wave morphology differs from sinus beats and varies depending on the origin of the impulse in the atria. If the ectopic focus is in the vicinity of the SA node, the P wave may closely resemble the sinus P wave (Figure 7-5). Its sole distinguishing feature may be its prematurity. As a rule, however, the P wave is different from the sinus P waves. In lead II (a positive lead), it’s generally upright and pointed (Figure 7-9), or it may be inverted (Figure 7-6) if the pacemaker site is near the AV junction. If the premature beat occurs very early, the abnormal P wave can be found hidden in the preceding T wave, causing a distortion of the T-wave contour (Figure 7-7).
The PR intervals of the PACs are usually normal, similar to those of the underlying rhythm. Occasionally the PR interval may be prolonged if the PAC is very early and finds the AV junction still partially refractory and unable to conduct at a normal rate. The PR interval will be unmeasurable if the abnormal P wave is obscured in the preceding T wave.
The QRS of the PAC usually resembles that of the underlying rhythm because the impulse is conducted normally through the bundle branches into the ventricles. The ventricles depolarize simultaneously, resulting in a normal duration QRS complex. If the PAC occurs very early, it is possible the bundle branches may not be repolarized sufficiently to conduct the premature electrical impulse normally. If the bundle branches are not sufficiently repolarized, the electrical impulse is conducted down one bundle branch (usually the left because it repolarizes quicker) and not conducted down the other. The left ventricle is depolarized first, followed by depolarization of the right ventricle (sequential depolarization). Sequential ventricular depolarization is slower, resulting in a wide QRS complex of 0.12 second or greater. A PAC associated with a wide QRS complex is called a PAC with aberrancy, indicating that conduction through the ventricles is abnormal (aberrant). Figure 7-8 shows a PAC with aberrant ventricular conduction (the QRS is wide) and a long PR interval, indicating conduction through the AV node was also delayed. Aberrantly conducted PACs must be differentiated from a premature ventricular contraction (PVC), especially if the abnormal P wave associated with the PAC is obscured in the preceding T wave. PVCs are discussed in Chapter 9.
The pause associated with the PAC is usually a noncompensatory pause (the measurement from the R wave before the premature beat to the R wave after the premature beat is less than two R-R intervals of the underlying regular rhythm) (Figure 7-9). This pause is called an incomplete pause because it doesn’t equal two R-R intervals. Less commonly, the PAC may occur with a compensatory pause (a pause that is equal to two R-R intervals), but this is usually seen with the PVC. The compensatory pause is called a complete pause because it equals two R-R intervals. To differentiate between a complete pause and an incomplete pause, the underlying rhythm must be regular. Rarely, the PAC may occur with a pause that is longer than compensatory.
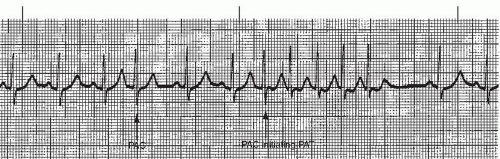
PACs may appear as a single beat (Figure 7-9), every other beat (bigeminal PACs, Figure 7-10), every third beat, (trigeminal PACs), every fourth beat (quadrigeminal PACs, Figure 7-11), in pairs (also called couplets, Figure 7-12), or in runs of three or more. Frequent PACs may initiate more serious atrial arrhythmias, such as paroxysmal atrial tachycardia (PAT), atrial flutter, or atrial fibrillation. Three or more beats of PACs in a row at a rate of 140 to 250 beats/minute constitute a run of PAT.
Premature atrial beats are common. They can occur in individuals with a normal heart or in those with heart disease. PACs may be seen with emotional stress (due to an increase in sympathetic tone), or ingestion of certain substances such as alcohol, caffeine, or tobacco. Other causes include hypoxia, electrolyte imbalances, myocardial ischemia or injury, atrial enlargement, congestive heart failure, and the administration of certain drugs, such as epinephrine or nonepinephrine, that increase sympathetic tone. PACs may also occur without apparent cause.
Infrequent PACs require no treatment. Frequent PACs are treated by correcting the underlying cause: reducing stress; reducing or eliminating the consumption of alcohol, caffeine, or tobacco; administering oxygen; correcting electrolyte imbalances; treating congestive heart failure, or discontinuing certain drugs. If needed, frequent PACs may be treated with beta blockers, calcium channel blockers, or antianxiety medications. Runs of PACs may require amiodarone to prevent more serious atrial arrhythmias from developing.
Occasionally, an ectopic atrial beat will occur late instead of early. This beat is called an atrial escape beat (Figure 7-13). Atrial escape beats usually occur during a pause in the underlying rhythm when the sinus node fails to initiate an impulse (sinus arrest) or when conduction of the sinus impulse is blocked for any reason (sinus exit block, nonconducted PAC, or Mobitz I second-degree AV block). The pause in the rhythm allows an ectopic pacemaker site in the atria to assume control of the heartbeat. The morphologic characteristics of the late beat will be the same as the PAC. Escape beats act as an electrical backup to maintain the heart rate and require no treatment.
Nonconducted PAC
A nonconducted PAC (Figures 7-14 7-15 and 7-16 and Box 7-3) results when an ectopic atrial focus occurs so early that it finds the AV node refractory and the impulse isn’t conducted to the ventricles. This results in a premature, abnormal P wave not accompanied by a QRS complex, but followed by a pause (Figure 7-14).
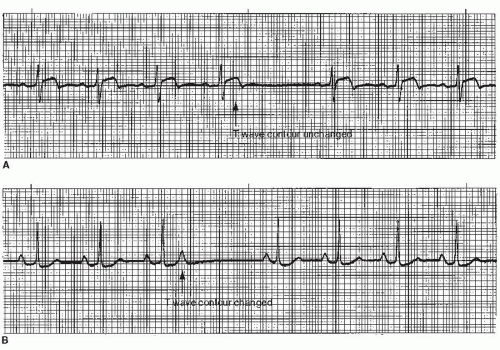
Like the conducted PAC, the P wave associated with the nonconducted PAC will be premature and abnormal in size, shape, or direction. The P wave is commonly found hidden in the preceding T wave, distorting the T-wave contour (Figure 7-15), and the pause that follows is usually noncompensatory.
Box 7-3. Nonconducted PACs: Identifying ECG features
Rhythm: Underlying rhythm usually regular; irregular with nonconducted PACs
Rate: That of underlying rhythm
P waves: P wave associated with the nonconducted PAC is premature, and abnormal in size, shape, or direction; often found hidden in preceding T wave, distorting the T wave contour
PR interval: Absent with nonconducted PAC
QRS complex: Absent with nonconducted PAC
The nonconducted PAC is the most common cause of unexpected pauses in a regular sinus rhythm. The nonconducted PAC can be confused with sinus arrest or block (especially if the P wave of the PAC occurs early enough to be hidden in the preceding T wave). All three produce a sudden pause in the rhythm without QRS complexes. To differentiate between these rhythms, one must examine and compare T-wave contours (Figure 7-16). The early, abnormal P wave of the nonconducted PAC will distort the preceding T wave. In sinus arrest or sinus block, no P wave is produced and the T-wave contour remains unchanged.
Nonconducted PACs have the same significance as conducted PACs and may be treated in the same manner.
Paroxysmal atrial tachycardia
Paroxysmal atrial tachycardia (PAT) (Figures 7-17 and 7-18 and Box 7-4) originates in an ectopic pacemaker site in the atria producing a rapid, regular atrial rhythm between 140 and 250 beats per minute. Atrial tachycardia is often precipitated by a PAC and commonly starts and stops abruptly, occurring in bursts or paroxysms (thus the name paroxysmal atrial tachycardia). By definition, three or more consecutive PACs (at a rate of 140 to 250 beats/minute) is considered to be atrial tachycardia (Figure 7-18). This rhythm may be due to enhanced automaticity of atrial pacemaker cells, resulting in rapid firing of an ectopic atrial focus, or to an atrial reentry circuit in which an impulse travels rapidly and repeatedly around a circular pathway in the atria.
Box 7-4. Atrial tachycardia: Identifying ECG features
Rhythm: Regular
Rate: 140 to 250 beats/minute
P waves: Abnormal (commonly pointed); usually hidden in preceding T wave, making T wave and P wave appear as one wave deflection (T-P wave); one P wave to each QRS complex unless AV block is present
PR interval: Usually not measurable
QRS complex: Normal (0.10 second or less)
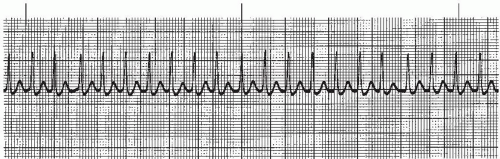 Figure 7-17. Paroxysmal atrial tachycardia. Rhythm: Regular Rate: 188 beats/minute P waves: Hidden PR interval: Not measurable QRS complex: 0.06 to 0.08 second. |
The P waves associated with atrial tachycardia are abnormal (commonly pointed), but may be difficult to identify because they’re usually hidden in the preceding T wave (the T wave and P wave appear as one deflection called the T-P wave). One P wave precedes each QRS complex, unless AV block is present. The PR interval is usually not measurable. The duration of the QRS complex is normal. Atrial tachycardia is characterized by regular, narrow QRS complexes, occurring at a rate of 140 to 250 beats per minute, and separated by the T-P wave.
Atrial tachycardia may occur in people with healthy hearts as well as those with diseased hearts. Atrial tachycardia has been associated with ingestion of substances such as caffeine, alcohol, or tobacco; anxiety; hyperthyroidism; use of drugs such as albuterol or theophylline; mitral valve disease; chronic obstructive pulmonary disease; and digitalis toxicity.
During an episode of atrial tachycardia, many individuals can feel the palpitations (rapid heart rate), and this is a source of anxiety. When the ventricular rate is rapid, the ventricles are unable to fill completely during diastole, resulting in a significant reduction in cardiac output. In addition, a rapid heart rate increases myocardial oxygen requirements and cardiac workload. Treatment of atrial tachycardia is directed toward controlling the ventricular rate and converting the rhythm.
Priorities of treatment depend on the patient’s tolerance of the rhythm. Cardioversion (synchronized electrical shock) is the initial treatment of choice in patients whose condition is unstable (patient is symptomatic with low blood pressure; cool, clammy skin; complains of chest pain or dyspnea; and exhibits signs of heart failure). If the patient’s condition is stable, sedation alone may terminate the rhythm or slow the rate. If sedation is unsuccessful, vagal maneuvers may terminate some episodes of PAT. Vagal maneuvers work by slowing the heart rate through increasing parasympathetic tone. Vagal maneuvers include coughing, bearing down (the Valsalva maneuver), squatting, breath-holding, carotid sinus pressure, stimulation of the gag reflex, and immersion of the face in ice water. If vagal maneuvers fail, administer a 6-mg bolus of adenosine IV rapidly over 1 to 2 seconds, followed by a rapid 10-mL flush of saline. If the initial dose is ineffective after 2 minutes, administer a 12-mg bolus of adenosine IV rapidly over 1 to 2 seconds, followed by a rapid 10-mL flush of saline. If the second dose is ineffective after 2 minutes, repeat a 12-mg dose of adenosine in the same manner.
If the patient doesn’t respond to vagal maneuvers or to the administration of three doses of adenosine, attempt rate control using a calcium channel blocker (such as diltiazem) or a beta blocker. These drugs act primarily on nodal tissue, either to slow the ventricular response by blocking conduction through the AV node or to terminate the reentry mechanism that depends on conduction through the AV node. In the setting of significantly impaired left ventricular (LV) function (clinical evidence of congestive heart failure or moderately to severely reduced LV ejection fraction), caution should be exercised in administering drugs with negative inotropic effects. These include beta blockers and calcium channel blockers, with the exception of diltiazem (a calcium channel blocker that exhibits less depression of contractility when compared with similar drugs).
When AV nodal agents are unsuccessful, cardioversion should be used to terminate the rhythm. Once the rhythm is terminated, antiarrhythmics may be effective in controlling the rhythm. Radiofrequency catheter ablation of the ectopic focus or reentry circuit is successful in many cases.