Fig. 7.1
The mid esophageal four chamber view represents a starting point in the evaluation of atrial septal defects as this cross-section displays both atria, the interatrial septum (IAS), atrioventricular valves, and ventricular inflows. LA left atrium, LV left ventricle, RA right atrium, RV right ventricle
Secundum ASDs, in general, are adequately visualized in this view with slight clockwise probe shaft rotation (Fig. 7.2, Video 7.2). Color flow Doppler interrogation in this plane demonstrates the presence and direction of shunting across the interatrial septum that can be verified by pulsedwave Doppler. This assessment also assists in the characterization of the flow across an interatrial communication in terms of the presence or absence of restriction (Fig. 7.3, Video 7.3). Slight probe withdrawal may be required to visualize small superior secundum defects, or probe advancement for small inferior secundum defects. Minimal probe shaft movements enhance the 2D evaluation allowing for examination of the defect in relation to adjacent structures as well as interrogation of the rims of the defect (Fig. 7.4). At times, smaller and/or more eccentric atrial defects will be detected in a standard TEE view (e.g. ME 4 Ch) only when a thorough sweep is performed of the septum. This emphasizes the importance of full sweeps when assessing communications at the atrial septum, as well as many other defects (Video 7.4).
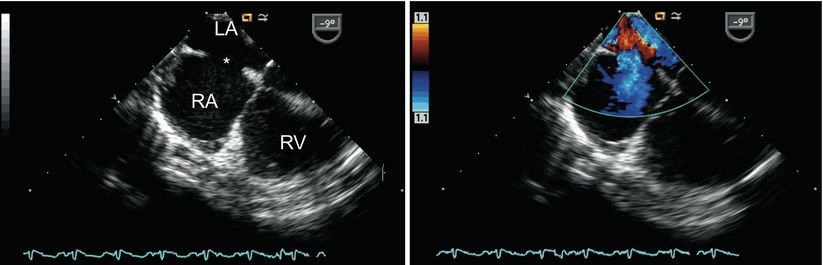
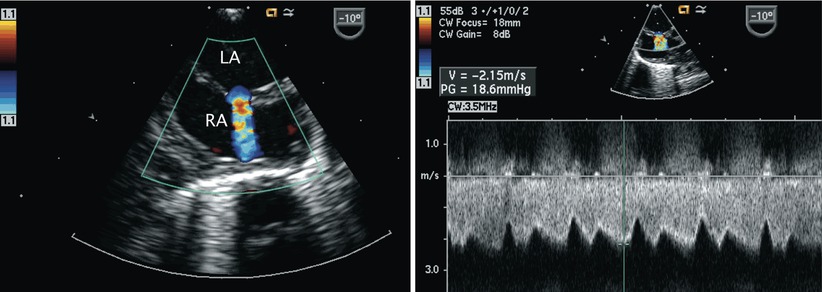
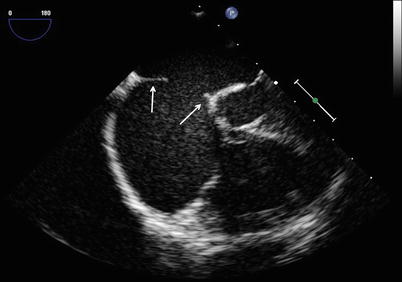

Fig. 7.2
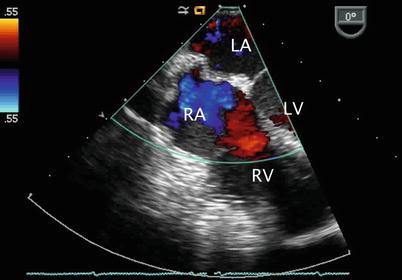
Left panel: Centrally located secundum atrial septal defect (asterisk) shown as seen in the mid esophageal four chamber view. Right panl: Color flow Doppler interrogation demonstrates left-to-right shunting across the defect (blue signal). Note that the probe has been rotated rightwards in this view to focus on the atrial communication. LA left atrium, RA right atrium, RV right ventricle

Fig. 7.3
Left panel: Image of the interatrial septum in the mid esophageal four chamber view with rightwards probe rotation. Note the aliased nature (mosaic of colors) of the jet across the atrial communication consistent with restriction across a small secundum atrial septal defect. The interatrial septum bulges in the rightwards direction suggesting elevated left atrial pressures. Right panel: Spectral Doppler interrogation confirms the restricted, high velocity flow across the defect with reduced phasic variation. LA left atrium, RA right atrium

Fig. 7.4
Image displays the rims (arrows) of a secundum atrial septal defect in the mid esophageal four chamber view with slight rightwards probe rotation
Progressive withdrawal of the probe in the ME 4 Ch view to above the level of the fossa ovalis demonstrates the superior aspect of the interatrial septum—the location of superior sinus venosus defects (Fig. 7.5, Video 7.5)—as well as the superior vena cava in cross-section. Anomalous drainage of the pulmonary veins as they enter the vena cavae can be seen from these views and attempts to visualize all veins should be made. The ME 4 Ch view also allows for 2D assessment of primum ASDs and Doppler interrogation of the atrioventricular valves (Fig. 7.6, Video 7.6; discussed in detail in Chap. 8).
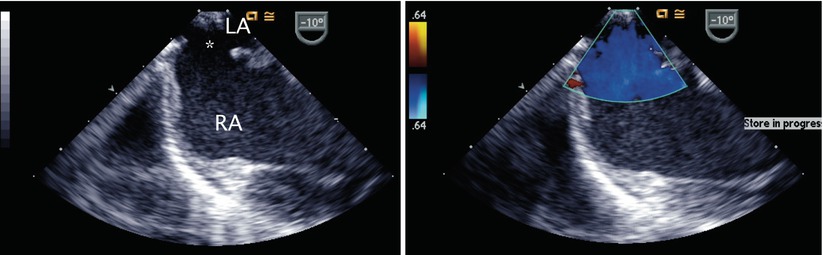

Fig. 7.5
Left panel: Superior sinus venosus atrial septal defect (asterisk) as seen by withdrawal of the imaging probe above the level of the fossa ovalis from a mid esophageal position. Right panel: Color Doppler imaging demonstrating flow across the defect. LA left atrium, RA right atrium

Fig. 7.6
Color Doppler image displaying left-to-right shunting across a primum atrial septal defect (asterisk) in the mid esophageal four chamber view. LA left atrium, LV left ventricle, RA right atrium, RV right ventricle
Clockwise turning of the probe in the ME 4 Ch view visualizes the remainder of the RA. The proximal aspect of the right atrial appendage (RAA) can be seen with slight probe withdrawal (Fig. 7.7, Video 7.7), in addition to portions of the tricuspid valve and right ventricular inflow (Fig. 7.8, Video 7.8) with clockwise probe shaft rotation. Tricuspid valve inflow and the degree of tricuspid regurgitation can be assessed from this window as well as in the mid esophageal right ventricular inflow-outflow (ME RV In-Out) and transgastric right ventricular inflow (TG RV In) views. Determination of the Doppler peak velocity of tricuspid regurgitation can be used to estimate right ventricular and pulmonary artery systolic pressures. Pulmonary artery diastolic pressure may be predicted by measurement of theend-diastolic velocity of pulmonary regurgitation from the ME RV In-Out, mid esophageal aortic valve short axis (ME AV SAX), or deep transgastric sagittal (DTG Sagittal) views. Increased tricuspid valve inflow and pulmonary outflow Doppler velocities may be seen within the context of large ASDs with significantly increased pulmonary to systemic blood flow (Qp:Qs) ratios. Increased flow across these structures does not necessarily represent valvar stenosis and in most cases are regarded as ‘flow-related gradients’. Pulmonary hypertension is quite rare, but may occur in patients with isolated ASDs over a period of many years.
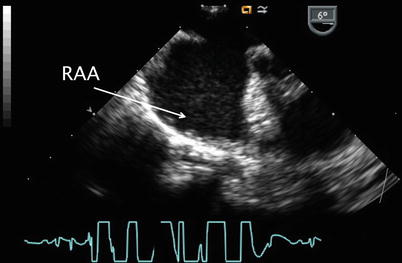
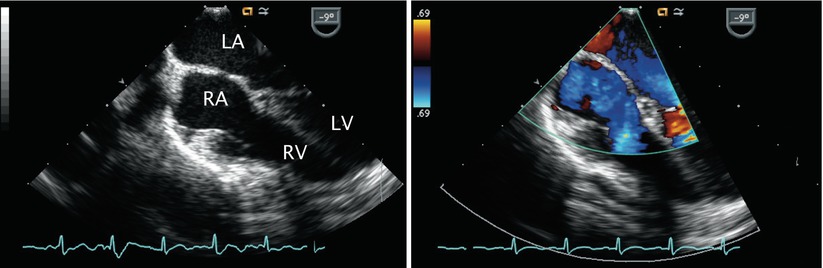

Fig. 7.7
Transesophageal image displaying the broad-based right atrial appendage (RAA). There is also a moderate to large secundum atrial septal defect present

Fig. 7.8
Left panel: Image obtained by turning of the imaging probe from the mid esophageal four chamber view clockwise to visualize right-sided structures. Right panel: Color Doppler demonstrates an intact atrial septum and laminar tricuspid inflow in this example. LA left atrium, LV left ventricle, RA right atrium, RV right ventricle
Returning to the ME 4 Ch view, sometimes requiring slight further clockwise turning, as well as advancement or withdrawal of the probe, connection of the right pulmonary veins to the LA posteriorly can be demonstrated (Figs. 7.9 and 7.10, Videos 7.9 and 7.10; also refer to Chap. 6).
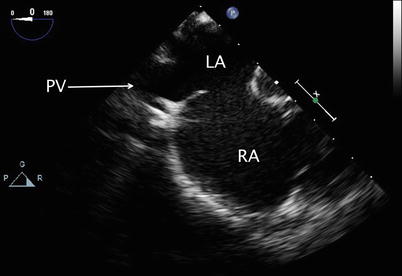
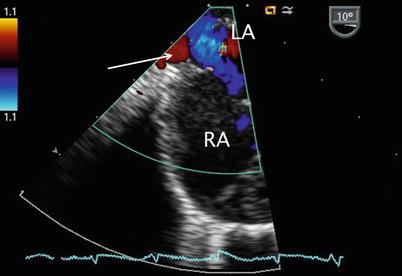

Fig. 7.9
Mid esophageal view with clockwise probe shaft rotation to examine the right pulmonary venous connections (PV) into the left atrium (LA). RA right atrium

Fig. 7.10
Color flow Doppler in a similar plane as shown in Fig. 7.9 to assess drainage of the right upper pulmonary vein (red color flow shown by arrow) into the left atrium (LA). RA right atrium
The sweep continues from the ME 4 Ch view with counterclockwise turning of the probe shaft to better visualize the LA (Fig. 7.11, Video 7.11). Probe advancement and retroflexion will enhance visualization of the coronary sinus and identify areas of unroofing, if present. Continued further counterclockwise rotation of the probe in conjunction with advancement or withdrawal demonstrates the left pulmonary venous connections to the left atrial posterior-lateral wall (Fig. 7.12, Video 7.12). Color and pulsed wave Doppler can be applied to facilitate this assessment (Fig. 7.13, Video 7.13). Pulmonary vein stenosis, although rare in most uncomplicated ASDs, may be suggested by aliasing/turbulence by color Doppler (Fig. 7.14, Video 7.14). This finding should be confirmed by spectral Doppler interrogation. This view may demonstrate a dilated coronary sinus in cross section if a persistent left superior vena cava to coronary sinus connection is present (Fig. 7.15, Video 7.15). Anterior to the entry of the left pulmonary veins with slight probe withdrawal to nearly a ME AV SAX plane, the proximal portion of the left atrial appendage (LAA) can be seen from this window (Fig. 7.16, Video 7.16). Forward axial rotation of the multiplane angle from the ME 4 Ch to the mid esophageal two chamber (ME 2 Ch) view (angle ~90°) displays the LA in an orthogonal plane and the LAA in long axis (Fig. 7.17, Video 7.17). Further counterclockwise turning of the TEE probe may visualize a left superior vena cava in long axis if one is present. From the ME 2 Ch plane forward transducer rotation displays a mid esophageal long axis (ME LAX) view (angle ~110°) and demonstrates the LA, mitral valve, and left ventricular outflow (Fig. 7.18, Video 7.18).
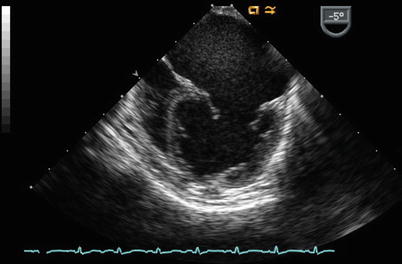
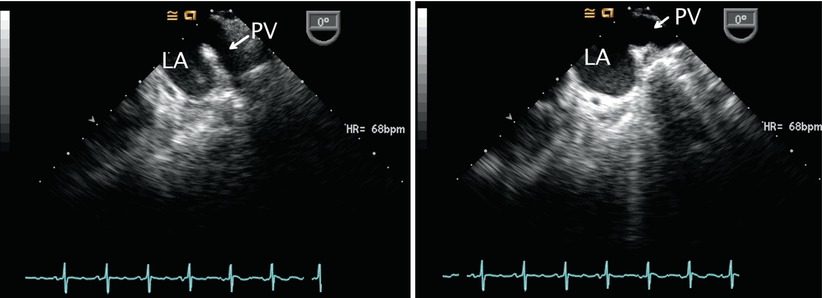
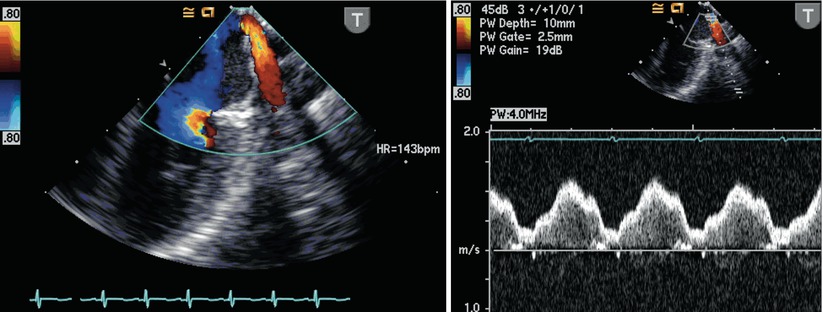
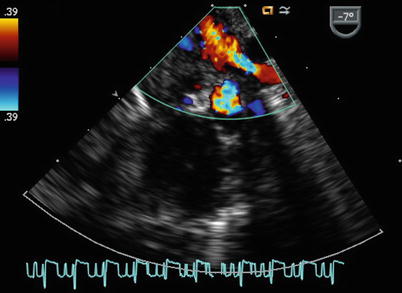
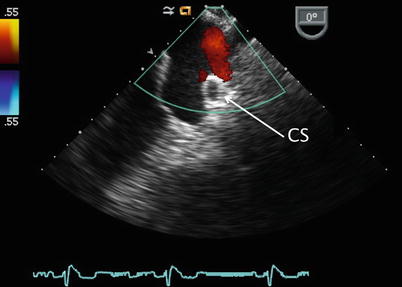
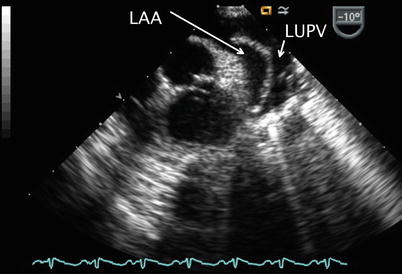
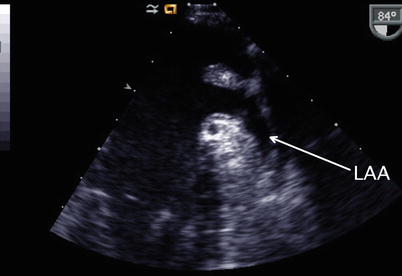
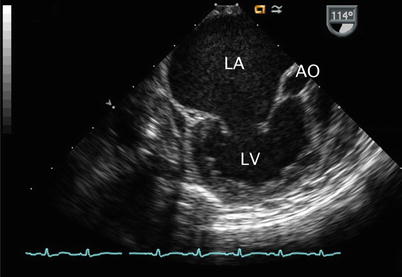

Fig. 7.11
Mid esophageal four chamber image obtained with counterclockwise rotation of the imaging probe demonstrates the left atrium (dilated due to mitral regurgitation in this example)

Fig. 7.12
The courses of two left sided pulmonary veins (PV) into the left atrium (LA) are demonstrated. Left panel: The left upper pulmonary vein is seen coursing in an anterior to posterior direction. Right panel: The more horizontal course of the left lower vein is demonstrated as it runs lateral to medial. Note the two different orientations of these veins as they enter the left atrium

Fig. 7.13
Color flow Doppler imaging (left panel) and spectral tracing (right panel) complements the two-dimensional evaluation of the pulmonary veins. Note the characteristic normal systolic and diastolic nature of the pulmonary venous flow

Fig. 7.14
Transesophageal color Doppler image of the left upper pulmonary vein as it courses towards the let atrium. Aliasing is seen by color flow interrogation that should prompt further investigation, including spectral Doppler interrogation

Fig. 7.15
Image displays a dilated coronary sinus (CS, arrow) as seen in cross-section (arrow) below the left pulmonary venous inflow. This may indicate the presence of a persistent left superior vena cava

Fig. 7.16
Image obtained with transducer anteflexion as this is slightly withdrawn to a nearly mid esophageal aortic valve short axis plane. This cross-section displays the left atrial appendage (LAA) anteriorly located in relation to the left upper pulmonary vein (LUPV)

Fig. 7.17
Mid esophageal two chamber view demonstrating the anterior position (arrow) of the left atrial appendage (LAA)

Fig. 7.18
Mid esophageal long axis view (multiplane angle 114°) corresponding to same patient displayed in Fig. 7.11. This image confirms the large dimensions of the left atrium (LA) and displays the left ventricle (LV) and aortic root (AO).
Slight advancement of the probe in the ME 4 Ch will allow for visualization successively of the coronary sinus as it courses longitudinally along the posterior atrioventricular groove (Fig. 7.19, Video 7.19), along with the coronary sinus orifice as it normally drains into the RA, and the lower portion of the interatrial septum. This is also the area where deficiency of atrial septal tissue surrounding the coronary sinus orifice on the RA may be identified in patients with a coronary sinus-type ASD. A dilated coronary sinus should not be confused for a primum ASD. Agitated saline injection into a left arm vein or left jugular vein will demonstrate contrast in the coronary sinus and drainage into the RA if a persistent left superior vena cava to coronary sinus connection is present (Fig. 7.20, Video 7.20) (Chap. 6).
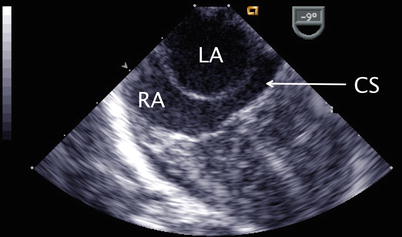
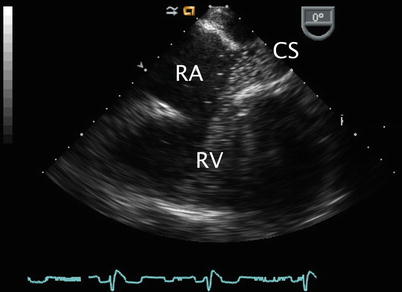

Fig. 7.19
Image obtained after slightly advancing the probe in the mid esophageal four chamber view. The coronary sinus (CS) in seen in long axis as it courses along the atrioventricular groove to drain into the right atrium (RA). LA left atrium

Fig. 7.20
Injection of agitated saline into a left arm vein in a patient with a persistent left superior vena cava demonstrating contrast in the coronary sinus (CS) thus confirming the connection. RA right atrium, RV right ventricle
From the ME 4 Ch view, clockwise turning of the probe and forward angle rotation displays the mid esophageal bicaval (ME Bicaval) view, angle ~90°–110°. This provides an excellent cross-section of the entire atrial septum (superior to inferior) (Figs. 7.21 and 7.22, Videos 7.21 and 7.22). The majority of the interatrial septum will be oriented optimally with respect to 2D and Doppler imaging from this view. This window is ideal for confirming the presence of superior (Fig. 7.23, Video 7.23) and inferior sinus venosus defects, secundum defects (Figs. 7.24, 7.25, and 7.26, Videos 7.24, 7.25, and 7.26), and a PFO [42–44]. As mentioned, contrast echocardiography with a Valsalva (or simulated Valsalva maneuver during mechanical ventilation) may be required for the detection of shunting across a PFO.
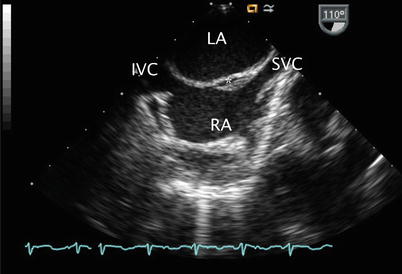
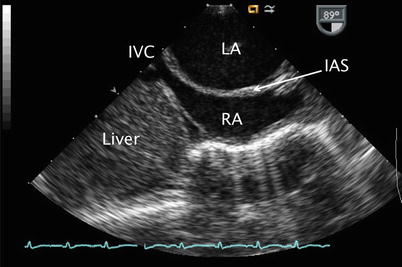
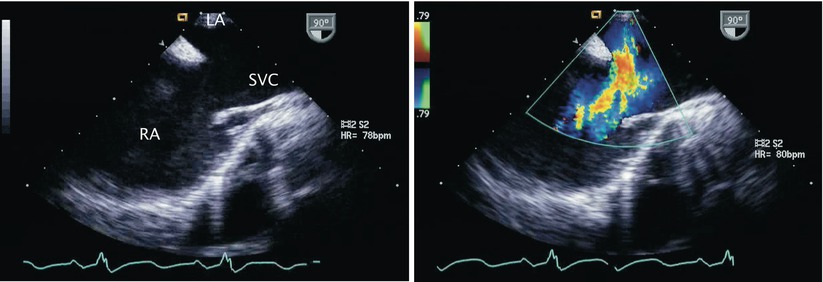
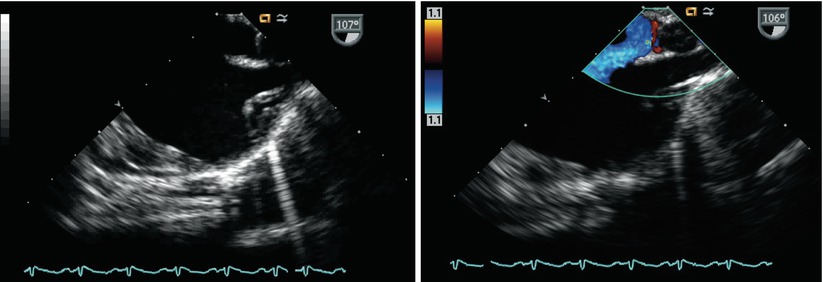
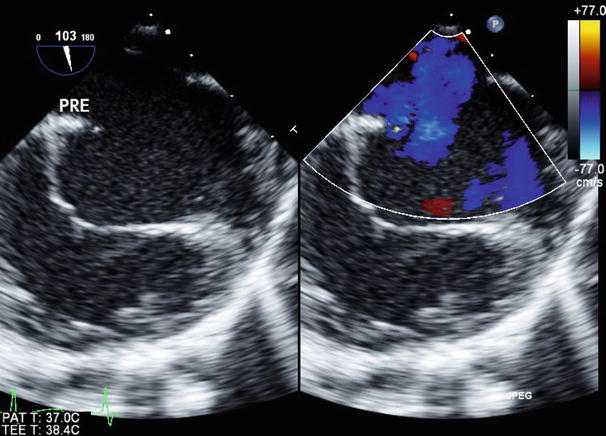

Fig. 7.21
Mid esophageal bicaval view displaying the entire atrial septum. The flap valve of the foramen is well seen (asterisk). IVC inferior vena cava, LA left atrium, RA right atrium, SVC superior vena cava

Fig. 7.22
Image of a mid esophageal bicaval view displaying a dilated left atrium (LA) with bulging of the interatrial septum (IAS) towards the right atrium (RA). The inferior vena cava (IVC) is also seen in this view it enters the RA

Fig. 7.23
Left panel: Two-dimensional image of a superior sinus venosus defect in the mid esophageal bicaval view. The superior vena cava (SVC) frequently straddles the interatrial septum in this defect. Right panel: Color flow Doppler confirms left-to-right shunting across the atrial communication. LA left atrium, RA right atrium

Fig. 7.24
Small secundum atrial septal defect as shown in the mid esophageal bicaval view

Fig. 7.25
Images demonstrate a high secundum atrial septal defect in the mid esophageal bicaval view (left panel) and corresponding color flow Doppler (right panel)

Fig. 7.26
Large secundum atrial septal defect (left panel) with associated left-to-right shunting (right panel). Note the slightly more inferior location of this defect as compared to the communication illustrated in a similar imaging plane in Fig. 7.25
Slight clockwise/counterclockwise probe turning from the ME Bicaval view may be necessary to optimally visualize an ASD depending upon its size and location. Withdrawal of the probe while in this view displays the superior vena cava in long axis. Probe advancement provides long axis imaging of the inferior vena cava (Fig. 7.22, Video 7.22). Anomalous pulmonary veins entering either vena cavae may be seen from these views with appropriate probe withdrawal and/or advancement (Fig. 7.27, Video 7.27)
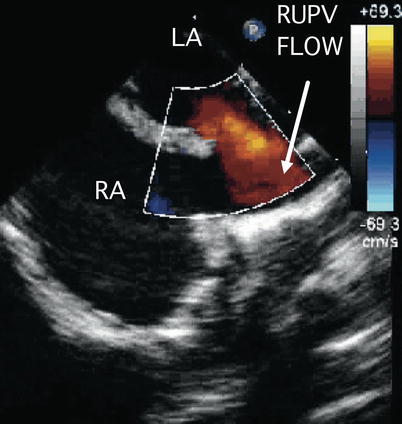

Fig. 7.27
Image demonstrates flow from the right upper pulmonary vein (RUPV) directed towards the superior vena cava-right atrial junction in a superior sinus venosus defect. LA left atrium, RA right atrium. Image courtesy of Thomas M. Burch, MD
While in the ME 4 Ch view, advancement of the probe displays the lower esophageal situs short axis (LE Situs SAX) view. The junction of the inferior vena cava into the RA and upper portion of the vena cava can be seen in this cross-section. Further clockwise turning of the probe may be necessary during this sweep. This view can also be useful in the assessment of inferior vena cava-type of sinus venosus defects and anomalous pulmonary veins entering the vena cava.
Forward axial rotation of the imaging probe through the ME RV In-Out view (angle ~60°), allows more complete anatomic assessment of the tricuspid valve, right atrial, and ventricular size, and right ventricular outflow tract and pulmonary valve. This view is particularly helpful to demonstrate right ventricular volume overload and systolic function (Fig. 7.28, Video 7.28) in the setting of communications at the atrial level. The transgastric mid short axis (TG Mid SAX) view also provides information regarding right ventricular size and magnitude of atrial level shunting in these lesions (Fig. 7.29, Video 7.29).
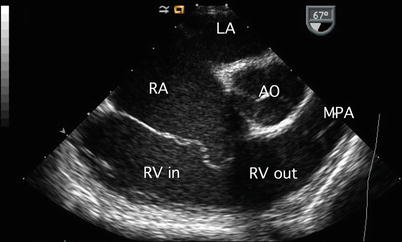

Fig. 7.28
Image of a mid esophageal right ventricular inflow-outflow view. This cross-section is useful in the comprehensive assessment of atrial septal defects as it displays the tricuspid valve, left atrium (LA), right atrial (RA) and right ventricular (RV) sizes, right ventricular inflow (RV in) and outflow (RV out) tract, pulmonary valve, and proximal main pulmonary artery (MPA). AO aorta

Fig. 7.29
Transgastric mid short-axis view demonstrating a large, volume loaded right ventricle (RV) in a patient with a large atrial septal defect. LV left ventricle
Further views of the interatrial septum can also be obtained from the deep transgastric window. Once the DTG Sagittal view is obtained (angle ~90°–100°) clockwise probe rotation as necessary allows for the entire interatrial septum to be displayed; the multiplane angle can be adjusted for optimal visualization of the atrial septum (Figs. 7.30, 7.31, and 7.32, Videos 7.30, 7.31, and 7.32). The deep transgastric windows complement the assessments of ASDs (Fig. 7.33, Video 7.33) and in some cases allow for details of the anatomy not visualized in other views to be demonstrated.
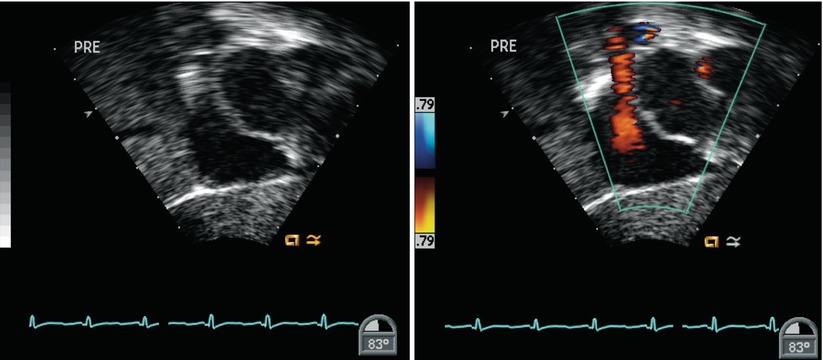
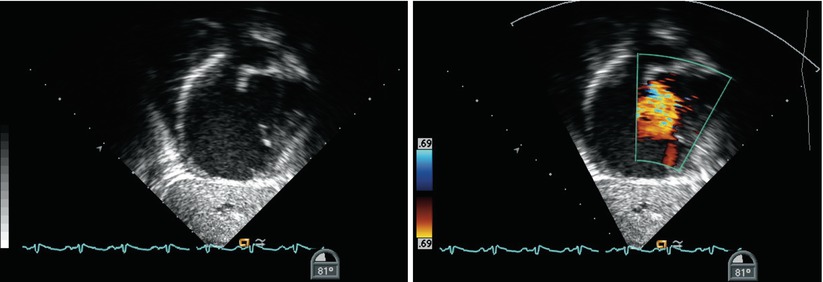
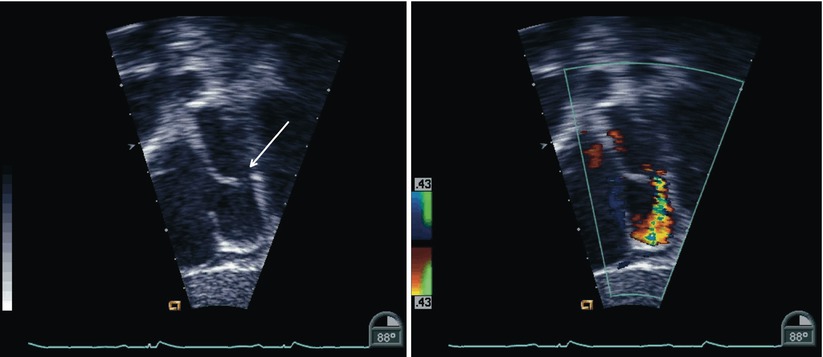

Fig. 7.30
Deep transgastric sagittal images of the atrial septum displaying two-dimensional (left panel) and color information (right panel). The interatrial septum appears intact by two-dimensional imaging

Fig. 7.31
Images obtained in the same cross-sections as shown in Fig. 7.30. In this example, the presence of a secundum atrial septal defect is demonstrated

Fig. 7.32
The image displays a modified plane in the deep transgastric window that also allows for echocardiographic assessment of the interatrial septum. LA left atrium, RA right atrium

Fig. 7.33
Images of an inferior sinus venosus defect as obtained from a modified deep transgastric sagittal view (same view as shown in Figs. 7.30 and 7.31). Note the deficiency (arrow) in the inferior aspect of the interatrial septum (left panel) and the presence of left-to-right shunting across this region (right panel). The Eustachian valve is also seen. Additional planes in this window may provide information regarding associated anomalies of pulmonary venous drainage
Evaluation of right atrial and right ventricular size, which generally reflect the relative volume of left-to-right shunting through an ASD, provides a qualitative assessment of the clinical significance of the defect. Pertinent TEE windows include: at the mid esophageal level the 4 Ch, RV In-Out and Bicaval views (Fig. 7.34, Video 7.34); at the transgastric level the RV In, Basal SAX, and Mid SAX views; and at the deep transgastric window the DTG Sagittal view and modified cross-sections. Qualitative assessment of right and left ventricular systolic function and color and spectral Doppler evaluation of associated lesions, as appropriate, are also indicated. Normal biventricular systolic function with flattening of the interventricular septum in diastole is a common finding in defects resulting in right ventricular volume overload.
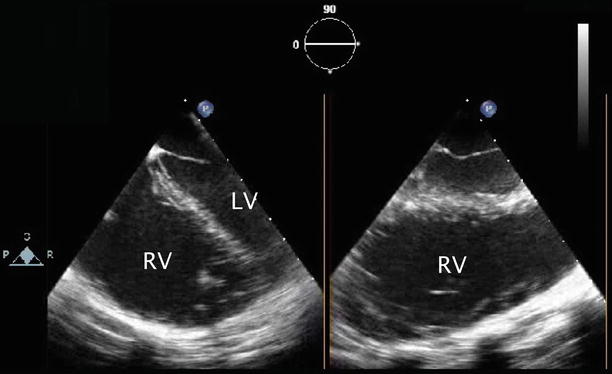

Fig. 7.34
Mid esophageal images in orthogonal planes that assist in the determination of right ventricular (RV) size and function. Moderate right ventricular dilation is shown. (LV) left ventricle
The considerations in the echocardiographic assessment of atrial level communications are summarized in Table 7.1.
Table 7.1
Atrial communications
Summary of important considerations for TEE assessment |
|---|
Anatomy of the atrial septal defect or patent foramen ovale |
Size and location of defect (single versus fenestrated) |
Septal rims |
Septal anatomy |
Direction of atrial level shunt |
Drainage of pulmonary veins |
Atrial chamber size |
Doppler assessment of atrioventricular valves (regurgitation); mitral valve anatomy (prolapse, cleft) |
Assessment for possible pulmonary hypertension |
Estimation of right ventricular systolic pressure by tricuspid regurgitation jet velocity |
Estimation of right ventricular diastolic pressure by pulmonary regurgitation end-diastolic velocity |
Identification and evaluation of other associated congenital cardiac lesions |
Ventricular chamber size and systolic function |
Surgical and Transcatheter Considerations
Preprocedure Assessment
Preoperative TEE evaluation focuses on confirming the size and location of the ASD, exclusion of additional congenital cardiac lesions, and assessment of atrial and ventricular chamber size and ventricular systolic function. Pulmonary venous anatomy should be evaluated, particularly in patients with sinus venosus ASDs. Care should be taken not to mistake a redundant Eustachian valve in the RA for the atrial septum and potentially misdiagnose an ASD that does not exist. Likewise, as discussed, a dilated coronary sinus should not be interpreted to represent a primum-type communication. Doppler evaluation of the atrial shunt and quantitation of the degree and peak velocity of tricuspid regurgitation should be performed.
In patients with a secundum ASD or PFO deemed suitable for transcatheter device closure, TEE evaluation includes size and location of the communication, evaluation of adequacy of atrial septal tissue rims (particularly the retroaortic rim), exclusion of additional septal defects, evaluation for atrial septal aneurysm (Fig. 7.35, Video 7.35), and assessment of ventricular chamber size and function [45–50]. The relationship of the defect to the vena cavae and atrioventricular valves and balloon sizing of the ASD or tunnel length of the PFO for suitable device selection are also important features to be evaluated. These considerations are discussed in detail Chap. 17.
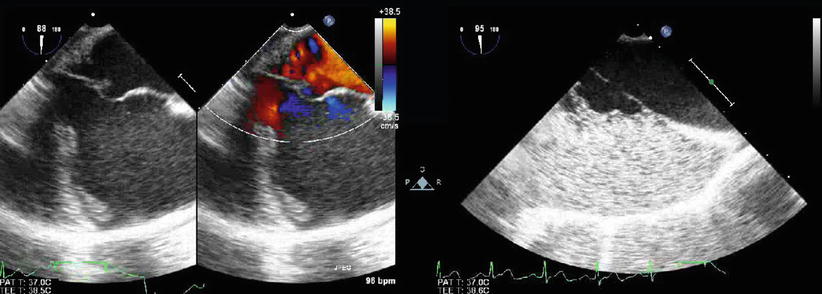

Fig. 7.35
Images demonstrate an aneurysm of the interatrial septum as shown by two-dimensional imaging (left panel), color flow mapping (middle panel), and contrast echocardiography (right panel). Injection of agitated saline into a lower extremity vein was performed to assess for the presence of a patent foramen ovale in a child with a history of multiple strokes
For some severe forms of CHD, an ASD is actually necessary to maintain a physiology compatible with survival. In these types of patients, restrictive or non-existent atrial communications may require catheter-based interventions. These are aimed at either creating or enlarging an atrial communication allowing for either enhanced mixing of systemic and pulmonary venous blood (e.g. d-transposition of the great arteries) or decompression of venous return typically in cases of atrioventricular valve stenosis or atresia (Fig. 7.36, Video 7.36). These interventions might also benefit from TEE guidance, particularly in infants, as this patient group may be quite ill. TEE may limit radiation exposure and enhance the safety of these procedures.
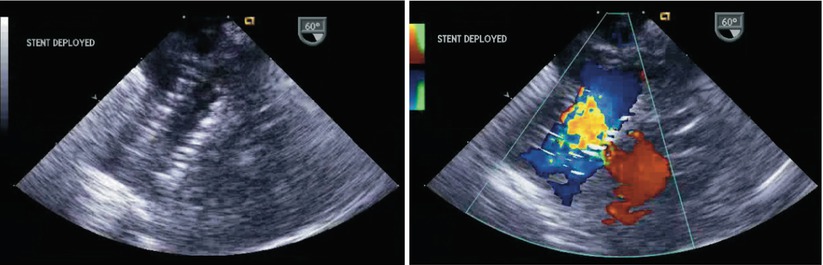

Fig. 7.36
Left panel: Two-dimensional image of an atrial septal stent placed following radiofrequency perforation of the interatrial septum in a critically ill infant with hypoplastic left heart syndrome and an intact atrial septum. Right panel: Color Doppler depicts flow across the central aspect of the stent
Postprocedure Assessment
Postoperative evaluation following surgical closure of an ASD begins with confirmation of adequate cardiac de-airing. Evaluation of the surgical repair involves exclusion of residual atrial level shunting (Figs. 7.37 and 7.38, Videos 7.37 and 7.38), quantification of atrioventricular valve regurgitation, and assessment of ventricular systolic function following weaning from cardiopulmonary bypass. In surgeries involving closure of sinus venosus ASDs with redirection of anomalous pulmonary veins it is very important to exclude vena caval or pulmonary venous obstruction post repair [51–53].