Fig. 8.1
Human carotid plaques stained histologically for neutral lipids (seen in red, Oil Red O). (a) A stable plaque with a thick cap and low lipid content. (b) An unstable plaque, rich in lipids, with thin cap and with luminal thrombi. Scale bars 500 μm (Courtesy of Dr. Andreas Edsfeldt)
1.
Large inflammatory activity with agglomerates of macrophages. High macrophage content has been suggested with more than 25 cells per 0.3 mm diameter of the plaque field [6].
2.
3.
Superficial erosion with possible endothelial damage or dysfunction, leading to increased attachment of inflammatory cells, deficient vasodilation and enhanced platelet aggregation.
4.
Fissured caps as lateral tears that lift a part of the intimal layer, exposing the thrombogenic core. These may stand for 15 % of the acute coronary deaths [9].
5.
High grade of stenosis. Even though most of the culprit lesions causing the symptoms do not show a significant grade of stenosis, a high-grade stenosis is a marker for advanced disease and for the presence of more plaques [10].
6.
Calcified nodule located close to the lumen, as it is highly thrombogenic.
7.
Intraplaque haemorrhage, often arising from friable neovessels that are quickly formed as a response to the hypoxic and necrotic environment of the core. These neovessels and haemorrhages are common in advanced plaques and are associated with the expansion of the core. Additionally, the erythrocyte debris increases the oxidative burden and cholesterol crystal amount, further perpetuating inflammation [11]. Intraplaque haemorrhage has been considered an independent predictor for cardiovascular events [12].
8.
Outward or positive remodelling, as the increase of size is towards the adventitia of the vessel. It is an early sign of the disease, considering that only when more than 40 % of the diameter is reduced does the lumen start to be affected by the following negative remodelling [13]. Plaques with more inwards or negative remodelling have more lipids and macrophages than those with positive remodelling.
Besides these characteristics, there are certainly many more that have been tested and are to be found. Nevertheless, it would be a major clinical gain if we could detect at least some of these properties in plaques before they cause events. Several approaches can be taken, using, for instance, circulating biomarkers, certainly a cheaper approach, but demanding large amounts of individuals to be cost-effective, or using imaging techniques, more expensive but potentially more specific, ideal for subgroups of risk stratification.
8.3 How Can the Characteristics of the Vulnerable Plaques Be Assessed?
We will focus initially on intravascular invasive methods and finally move on to the three most common non-invasive methods used for plaque characterization:
8.3.1 Intravascular Ultrasound (IVUS), Optical Coherence Tomography (OCT) and Near-Infrared Spectroscopy (NIRS)
IVUS uses a transducer on the end of a flexible, steerable catheter inside the arteries allowing ultrasound of the vessels from the inside out. It is routinely used clinically to delineate plaque morphology, lesion length and obstruction severity when coronary angiography and/or pressure data are ambiguous. Additionally, it may help to guide percutaneous coronary interventions and detect in-stent restenosis. IVUS allows the actual visualization of the plaques – which the angiography actually does not allow, as it is simply a lumenography.
The main pitfalls of IVUS are its invasiveness with its inherent risks, high costs and being time-consuming and the fact that it demands particularly trained human resources. Additionally, only a segment of the coronary tree is assessed at a time, and there is no consensual direct evidence linking changes in the coronaries’ plaque and clinical events. Studies to assess this would have to include large numbers of subjects to be then followed up with a non-risk-free technique. Nevertheless, IVUS has been widely used in smaller cohorts to test statin effects [14–16], as well as other therapies [17], with particular focus on the volume and size of the atheroma [18, 19].
Deriving from IVUS, several applications have been developed from the different manufacturers, such as virtual histology IVUS (Volcano Therapeutics), iMAP (Boston Scientific), integrated backscatter IVUS and automated differential echogenicity. Focusing again in plaque composition, tissue maps have been created that correspond to four major components: fibrous (green), fibrofatty (light green), dense calcium (white) and necrotic core (red) [20, 21]. Calcium appears as bright echoes with acoustic shadowing, as dense calcium obstructs the penetration of ultrasound. Therefore, IVUS detects only the leading edge and not the thickness of the calcification. However, it is a good technique to assess remodelling, both positive and negative. Thrombus, either fresh or organized, is the ultimate pathological feature leading to acute events. Unfortunately, none of the IVUS-based imaging modalities available can reliably identify thrombus as it varies largely in its aspect. Recently, there have been attempts to create scores that would distinguish culprit lesions and plaques causing stable angina, such as the Liverpool Active Plaque Score, which was based in a combination of plaque characteristics, namely, core and calcium ratio, minimum lumen area, remodelling index and thin-cap fibroatheroma [22].
More recently, the validation of some of these approaches has been questioned [23], which together with the pitfalls mentioned above contributed to the partially decreased interest in these approaches in many centres.
OCT is a fast-acquisition technique that has submicron resolution, at the expense of reaching very small depths, such as 1–2 mm. It is based on an optical beam that is directed at the tissue. A small part of this light reflects from features under the surface and is collected back. The light is in the near-infrared range, not visible to the human eye. No radiation is involved. Its major advantage is the very high resolution, ca ten times better than IVUS. OCT does not allow visualization through blood so it has to be flushed from the field of view, which in itself can cause complications. Just like IVUS, it is an invasive, catheter-based technique, with the subsequent risks and cost.
In atherosclerosis, it became particularly popular to study the coronaries, providing images of the fibrous cap [24, 25], particularly if ruptured and for very detailed assessment of stents [26]. OCT is quite good for assessing thrombus and even to distinguish red (red blood cell-rich) thrombus from white (platelet-rich) thrombus [26]. A thin cap and thrombosis are two important characteristics of VP. On the other hand, other characteristics are more challenging to detect. Macrophages may only be seen sometimes if in larger agglomerates. The lipid or necrotic core is a signal-poor region within the plaque, with poorly delineated borders [25, 26].
Potentially better to assess plaques with lipid-rich cores is NIRS. NIRS, as its name reveals, is also an optical technique that uses the near-infrared region of the electromagnetic spectrum. NIRS is actually widely used to determine the chemical content of substances. First tested in autopsies [27], NIRS could detect lipid pool, thin fibrous cap and inflammatory cells in human aortic atherosclerotic plaques. Soon after, the catheter-based spectroscopy system was implemented further, using wavelengths that can penetrate blood, fast enough to cope with cardiac motion. This system became particularly popular to identify lipid core coronary plaques in patients [28]. Results are usually depicted as chemograms – coloured maps showing the probability of the presence of lipid core plaques. Further studies are needed to establish the possible clinical role of NIRS. Nevertheless, it is an invasive technique with its implicit risks and costs.
8.3.2 Ultrasound
Ultrasonography is based on the emission of ultrasound waves that have higher frequency than 20 kHz (maximal frequency that can be heard by humans). Ultrasounds generated by the transducer propagate through the tissues and, depending on the tissue composition, can be partly reflected in different structures. They are then received back as vibratory mechanical energy, which is finally converted into electric signals and presented as images on the screen. The different acoustic impedance of tissues allows the detection of acoustic interfaces, allowing the definition of their echogenicity. The bigger the impedance differences between two tissues, the better the ultrasonographic discrimination between them. High emitted frequency allows a better definition of the structures that are more superficial. With ultrasound, distances can be quantified by measuring the time between transmission and reception of the signal, as the velocity of propagation of ultrasound in soft tissue can be considered constant (1,540 m/s) [29].
Ultrasonography, which is not intravascular, is a non-invasive method, without known toxicity or radiation. It can be done quickly at the “bedside”, and it is cheap when compared with other techniques. Moreover, it allows both functional and morphological evaluations of the whole vessel wall, in contrast to angiography where only the lumen is outlined. It has become widely used as a standard diagnostic modality for carotid stenosis evaluation. No technique is totally perfect, and carotid ultrasonography may have limitations in discriminating subtotal from total occlusion in assessing plaques that have an acoustic shadow and in patients with short neck, high carotid bifurcation or severe arterial kinking. Operator dependency has been the most important limitation of ultrasonography.
Several subjective classifications for the ultrasonographic aspect of plaques have been proposed along the years. Gray-Weale et al. [30] divided plaques in echolucent, predominantly echolucent, predominantly echogenic and echogenic ones, inspiring other adaptations of this classification.
However, the need for objective criteria was more and more urged. Thus, standardization methods to process plaque images were created to overcome differences in the evaluation of plaque echogenicity between the different equipments and observers. One of the most used standardization methods was based on the greyscale values of pixels in a scale of grey intensities (0 darkest and 255 brightest). They used the greyscale median (GSM) as a score of global echogenicity. The reference values were the blood as 0 (the darkest structure in the image) and the adventitia as 190 (the brightest structure in the image). The whole plaque image outlined would be processed by linear scaling, compressing the scale. Making the blood and adventitia of all the images have the same values allowed the comparison of different plaques, assessed with different equipments, set-ups and observers. Intra- and interobserver variabilities improved considerably [31].
Plaques with low GSM have been associated with higher incidence of cerebral infarction [32, 33]. Gronholdt et al. [34] showed that echolucent plaques causing stenosis >50 % are associated with increased risk of stroke in symptomatic but not asymptomatic individuals. The association between echolucency and high risk for ipsilateral symptoms has also been verified in other studies, though the influence of the degree of stenosis was not always accepted [35, 36]. Whereas some suggested hypertension and progressive lesions to be important additional determinants of risk [37], others proposed that echolucent plaques were associated with increased risk of neurological events, even independently of degree of stenosis and cardiovascular risk factors [38].
Some other studies evaluated heterogeneity of the plaque image and found that heterogenous and/or echolucent plaques are associated with higher risk of stroke [39–44]. The ability of ultrasonography to study other characteristics that could be associated with the presence of symptoms has been intensively studied. The identification of ulceration by ultrasonography in different studies showed large variation. Rubin et al. [45], using ultrasonography, detected 93 % of the ulcerated lesion, whereas Comerota et al. [46] argued that the possibility of detecting ulceration varied with different degrees of stenosis, being particularly difficult in high-grade stenoses. Bassiouny et al. [47] found that the proximity of plaque necrotic core to the lumen is associated with clinical ischaemic events. Our group [48] showed that in heterogenous plaques, juxtaluminal location of the echolucent region was associated with increased risk (Fig. 8.2). On the contrary, in homogenous plaques, the absence of an echogenic cap and disruption of the plaque surface correlated with symptoms. In an attempt to determine the relative importance of the ultrasonographic structural characteristic of the plaques, besides GSM, an activity index was calculated. This activity index was associated with symptoms [49]. The parameters associated with the presence of ipsilateral symptoms were surface disruption, severe stenosis and low GSM and, in heterogenous plaques, the presence of a juxtaluminal echolucent area. Though all these results are interesting, large-scale studies on different ultrasonographic aspects that can reflect risk and on the natural history of the plaques are needed.
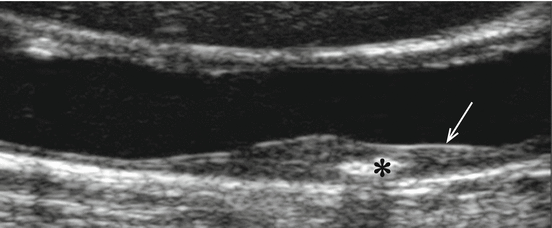

Fig. 8.2
Human carotid plaque, longitudinal image, with B-mode ultrasound. The fibrous cap is shown by the arrow. The white region marked with an * is calcified, causing a shadow cone
Although the association of echolucent plaques with higher neurological risk has become accepted, no ultrasonographic characteristics have been associated with a single type of symptom. The majority of the studies did not separate those end points. TIA patients showed more hypoechoic carotid plaques with longitudinal motion. The association between plaque radial and longitudinal motion and neurological events was demonstrated in other studies [50].
We [51, 52] and other groups [53–56] have characterized the composition of the echolucent plaques by being richer in lipids, necrosis, haemorrhage and inflammatory cells and poorer in calcification and fibrosis than the echogenic plaques. The presence of neovessels in the plaque branches from the vasa vasorum, which may lead to intraplaque haemorrhage, may be seen with ultrasound, particularly if using contrast [57].
8.3.2.1 Effects of Drug Interventions
Besides the very common use in clinical trials of the intima-media thickness, plaque ultrasound has also started to be used as a tool to monitor the effect of therapeutic interventions, such as statins [58–60] and beta-blockers [61], or even risk for carotid stenting [62]. Further studies on new algorithms for plaque characterization are warranted and will be of great use in risk stratification and to follow up interventions in a cheap, non-invasive, contrast- and radiation-free approach in the future.
8.3.3 Computed Tomography (CT)
CT is a widely spread technique, available in most institutions, with a higher spatial resolution than MR, freedom from flow-related artefacts and simple and fast acquisition, demanding less patient-dependent modifications. The newer machines with multiple detectors and ECG triggering require very small timings of acquisition and need only short injections of contrast.
CT major pitfalls besides radiation and contrast are metal artefacts and, which concerns plaques in particular, calcifications. Calcifications, particularly if very large, may provide blooming artefacts. Other issues that might hamper the quality of the CT images are arrhythmias and fast heart rates, which motivate frequent use of beta-blockers before the scan.
Coronary calcification increases with age and is considered a marker of atherosclerotic burden [63], even though it does not reflect the rupture risk of individual plaques. There have been several approaches to quantify the calcium burden, but the one that became more popular and used in the clinical routine is the Agatston calcium score [64] – calculated by multiplying the lesion area by a weighted attenuation coefficient corresponding with the measured peak CT attenuation or by the volume and mass scores.
Nevertheless, it is now consensual in several position articles from the American Heart Association that coronary artery calcium correlates with greater overall severity of the atherosclerotic process and with the probability of coronary stenosis somewhere within the coronary circulation. Simultaneously, higher calcium scores are related to a higher overall risk for a future cardiac event. However, most interesting is the high negative predictive value for nonsignificant coronary disease of the absence of coronary calcium [65]. This makes CT an excellent method to rule out disease, particularly in patients with low to intermediate risk.
To evaluate the lumen as an angiography, CT is quite feasible, allowing good visualization of native vessels, coronary anomalies, chronic total occlusions, bypass grafts and even stents particularly if larger (>3 mm) [66]. However, as mentioned above, metallic clips and stent nets may cause artefacts creating interpretation difficulties.
CT may also be used for functional purposes, such as assessing the left ventricular function or even perfusion [67], but these aims can generally be obtained, at least as well, with MRI or ultrasound with the beneficial aspect of no use of radiation. More recently, it has become possible to assess the coronary flow reserve non-invasively with CT [68], which despite needing further validation is an extremely promising approach for the future.
Using contrast, it is possible to see both calcified and non-calcified plaques in the arterial tree (Fig. 8.3). More and more studies have tried to assess plaque composition with CT. Plaques in heart specimens with low densities consist mostly of lipids, while those with high densities have more fibrous tissue [69]. Thrombus may look like non-calcified lesions which may be a problem in the interpretation of plaque composition [70]. Carotid plaque CT signal attenuation correlates with the amount of calcification histologically and not with the lipids or haemorrhage [71]. Calcium scores in the carotids may represent an independent marker for luminal stenoses and symptoms [72], with lower calcium content associated with a greater prevalence of symptoms [73]. Hoffman et al. [74] showed that CT can detect non-invasively differences in lesion morphology and plaque composition between culprit lesions in acute coronary syndrome and stable lesions. The culprit lesions giving rise to acute coronary syndromes have larger areas, more remodelling and less calcified plaques than the stable lesions. Another particularly interesting study conducted by Motoyama et al. [75] demonstrated prospectively (follow-up of 27 months) that patients with positively remodelled coronary segments with low-attenuation plaques on CT angiography were at a higher risk for acute coronary syndrome.
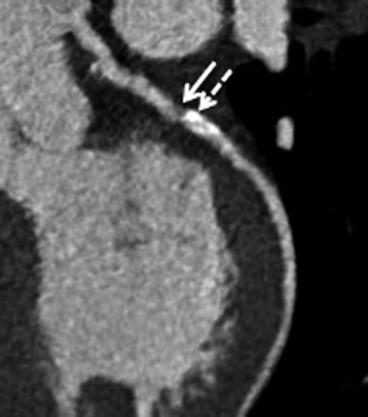

Fig. 8.3
Human coronary plaque in the left anterior descending artery, with a non-calcified part (filled arrow) and with a calcified part (dashed arrow), assessed with CT 64 detectors
A ringlike attenuation pattern of coronary plaques termed as napkin-ring sign was described in the CT of patients who had acute coronary syndromes. This sign was independently related to the size of the core and of the plaque and to the vessel area, measured histologically in seven autopsy specimens [76]. This concept was further reinforced by a recent longitudinal study [77] where the napkin-ring sign could predict future acute coronary syndromes, independent of the presence of positive remodelling and obstructive or low-attenuation plaques.
Taken together, one can recognize several of the characteristics of the VP by being now detected with CT.
8.3.4 Magnetic Resonance (MR)
MR has recently almost become the new gold standard for plaque composition, even though it might overestimate the degree of stenosis. Several protocols in varied field strengths and manufacturers have been implemented. For plaque characterization, dedicated coils have been developed.
The major pitfalls of MR are flow and motion artefacts as well as metal susceptibility artefacts. It is an expensive technique, both time-consuming and demanding a good degree of technical expertise for plaque characterization using multiple sequences. Many patients tend to feel claustrophobic and discomfort with the noise. For MR, nephrotoxic contrast may be used, which makes it less attractive for renal insufficiency or prior contrast reaction cases. Nevertheless, it may be slightly better for renal insufficiency patients than CT.
MR differentiates plaque components on the basis of biophysical and biochemical parameters, such as chemical composition and concentration, water content, physical state, molecular motion and diffusion. As it needs no ionizing radiation, it can be repeated over time, which has made it very attractive for clinical intervention trials where the imaging follow-up is of interest. Most of the in vivo studies have used a multi-contrast approach, using different sequences, allowing the detection of several components simultaneously.
In what concerns how MR can assess plaque composition, studies have been blooming in the last decade, particularly in the carotid territory (Fig. 8.4), as it is more accessible than the coronaries, but it has been tested at least for research purposes in all of these arterial beds. MR allows the identification of fibrous caps on carotid plaques, and their rupture has been related with recent stroke [78]. MR identifies calcium, lipid core, haemorrhage and fibrotic tissue with high sensitivity and specificity [79]. It has also been used to detect inflammation, correlating the transfer of contrast into the extracellular space with macrophages and loose matrix content [80]. Thrombus is a very important component related to plaque rupture or erosion and is sometimes challenging to image. MR has been a good technique to detect not only intraplaque haemorrhage but also juxtaluminal haemorrhage and thrombus [81–83]. Differences between symptomatic and asymptomatic plaques using MR have been found in the same patient [84]. As it is a very reproducible method, MR demands less patients in future clinical trials, being a promising technique to assess VP.
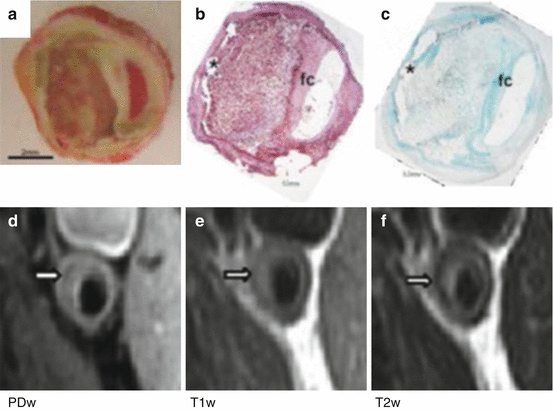

Fig. 8.4
Human carotid plaque: (a) macroscopic image, (b) macrophage immune staining (CD68) in dark red, (c) collagen staining (Masson) in green. The same plaque assessed in vivo with 3 T MR (d) proton density-weighted, (e) T1-weighted and (f) T2-weighted images. The dark basal rim (white arrow) corresponds to calcium marked with * in histology images. Fc fibrous cap
An interesting recent approach with MR has been the non-invasive measurement of temperature of the plaques in vivo, which may reflect plaque inflammatory activation [85]. The technical developments in MR are very fast and exciting including new weightings, new external coils, spectroscopy, targeted molecular imaging (using contrast agents linked to antibodies, peptides or iron oxide compounds, recombinant lipoproteins), higher fields of strength and wall stiffness, just to name a few.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


