There are limited data on the clinical significance of left ventricular (LV) mass and late gadolinium enhancement (LGE) in pediatric hypertrophic cardiomyopathy (HC). We reviewed cardiovascular magnetic resonance (CMR) studies of children with HC to investigate the associations between the extent and distribution of LGE and LV mass with ventricular tachycardia (VT) in children with HC. A blinded observer reviewed CMR studies for the presence and distribution of LV hypertrophy and LGE using a 17-segment model. The primary outcome was VT. LGE was present 17 of 33 subjects (52%). VT was present on outpatient Holter monitor or exercise stress test in 7 patients, of which 5 patients (71%) had LGE. Each additional segment of LGE was associated with an increase in the odds of VT (odds ratio [OR] 1.4, 95% CI 1.1 to 1.9) and fewer than 5 segments with LGE had 93% specificity for the presence or absence of VT (OR 0.06, 95% CI 0.01 to 0.5). VT was more common in patients with LGE in the apical septal (p = 0.03), basal inferoseptal (p <0.01), and basal inferior (p = 0.04) segments, whereas LGE in more commonly involved segments (midanteroseptal and midinferoseptal) was not associated with VT (p = 0.13, 0.26). Patients with VT had greater LV mass index (76.4 ± 40.4 g/m 2.7 vs 50.9 ± 24.3 g/m 2.7 ; p = 0.03). Each centimeter of increased maximum LV thickness was associated with increased likelihood of VT (OR 2.9, 95% CI 1.2 to 6.8). In conclusion, in pediatric HC, CMR to evaluate the extent and pattern of LGE, LV mass index, and maximum LV thickness may help to identify children with HC at risk of VT.
Cardiovascular magnetic resonance (CMR) with late gadolinium enhancement (LGE) has emerged as a marker of myocardial fibrosis and elucidating its role in sudden cardiac death (SCD) risk assessment has recently garnered significant interest. In the setting of hypertrophic cardiomyopathy (HC), myocardial fibrosis likely plays a role in the pathogenesis of life-threatening ventricular arrhythmias. CMR is widely accepted as a gold standard assessment of left ventricular (LV) mass and thickness, which have been associated with arrhythmic events in patients with HC. Although recent adult cohort studies have investigated the clinical utility of the presence and extent of LGE in HC, there are limited data on the pattern of LGE involvement. Furthermore, there are limited data on the clinical significance of LGE and LV mass in the pediatric population. We sought to investigate the association between CMR measurements of distribution and extent of LGE and LV mass with ventricular tachycardia (VT) in children with HC.
Methods
The institutional review board approved this study. We performed a retrospective review of CMR studies of children with HC performed at Texas Children’s Hospital as part of clinical care. Patients diagnosed clinically with HC during the study period were included. HC was defined as inappropriate LV hypertrophy diagnosed by echocardiogram. Patients with systemic hypertension, obstructive causes of LV hypertrophy, or incomplete medical records were excluded. Genetic testing for sarcomeric HC was performed in 8 patients; patients were therefore not stratified based on genetic diagnosis. Genotype-positive patients who did not carry the phenotype of HC were not included. All CMR studies were performed on a 1.5-T system (Achieva/Intera; Philips Medical Systems, Best, the Netherlands). In patients with multiple CMRs, the first CMR was used. Steady-state free precession acquisitions were obtained in the short-axis plane. Volumetric and phase contrast analyses were performed on an Extended MR WorkSpace workstation (Philips Medical Systems, Utrecht, the Netherlands). The endocardium and epicardium were traced, excluding the papillary muscles, on each of 12 to 14 end-diastolic and end-systolic frames. LV mass was calculated using preprescribed myocardial density. These techniques have previously demonstrated low interobserver variability in children. LV mass was indexed to height 2.7 to calculate LV mass index (LVMI).
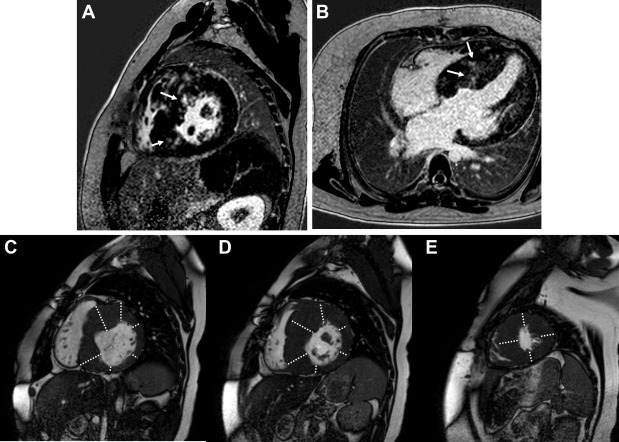
Assessment for LGE involved a 2-dimensional inversion recovery gradient echo sequence in the short-axis and 4-chamber planes performed 8 to 12 minutes after administration of single dose (0.1 mmol/kg) of gadopentetate dimeglumine (Magnevist; Bayer Pharmaceuticals, Whippany, New Jersey) for perfusion followed by 8 to 12 minutes after contrast administration. Studies performed earlier in our experience used a standard gradient echo recovery sequence for LGE assessment, whereas later in our experience, we used a phase-sensitive inversion recovery sequence. An expert observer (CVN) blinded to outcome assessed each of 17 LV segments for LGE ( Figure 1 ). Patients who did not undergo LGE evaluation or with uninterpretable images were excluded from LGE analysis. To assess for LV hypertrophy, the blinded observer measured each LV segment in the short-axis plane and compared values to published normative data of septal and free wall thickness ( Figure 1 ). The “apex” segment was measured in the 4-chamber plane. As robust, CMR-based pediatric normative standards for LV thickness do not exist, echocardiography-based data were used.
Parametric data are expressed as mean (±SD), whereas nonparametric data are expressed as median (interquartile range [IQR]). Associations between independent variables and the primary outcome, VT, were determined using univariate logistic regression. Sustained and nonsustained VT were included and were diagnosed on Holter monitor or exercise stress test. During the study period, the institutional protocol was to perform at least annual Holter monitoring and exercise stress tests, although those with significant left ventricular outflow tract obstruction were often limited from treadmill testing. Isolated ventricular contractions and couplets were not defined as VT. The medical record was reviewed for clinical outcomes, including other arrhythmia, syncope, heart transplant, and death. Continuous data were compared between groups using the t test or Mann–Whitney U test when appropriate. Categorical data were compared using the chi-square test. Receiver operating characteristic analysis was used to identify a cut-off value of the number of LV segments with LGE.
Results
A total of 39 children with HC underwent CMR at our institution from April 2005 to July 2012. Patient characteristics are described in Table 1 . Five patients (13%) had genetic syndromes that included Noonan Syndrome, Friedrich Ataxia, and LEOPARD syndrome.
| Patient Characteristics (n=39) | |
| Age at diagnosis (years) | 11.9 (4.0, 14.0) |
| Age at last follow up (years) | 15 ± 4.7 |
| Follow up time (years) | 4.5 (1.9, 7.8) |
| Age at MRI (years) | 13.2 ± 5.0 |
| Male | 30 (77%) |
| Genetic syndrome | 5 (13%) |
| Family history of HCM | 10 (26%) |
| Family history of SCD | 2 (5%) |
| Patient Outcomes (n=39) | |
| Ventricular tachycardia | 8 (21%) |
| ICD implanted | 7 (18%) |
| Myectomy | 7 (18%) |
| Aborted sudden cardiac death | 1 (3%) |
| Transplant | 1 (3%) |
| Death | 0 |
VT occurred in 8 patients (21%), in 2 before the performance of the CMR and in 6 patients subsequent to the CMR, at a median of 17 months (range 4.6 to 38). One patient experienced aborted SCD attributed to VT. No patient died during the study period. Indications for ICD placement in this cohort included VT with or without other classic risk factors. The presence of VT was not associated with age at diagnosis of HC, follow-up time, gender, positive family history of HC, or the presence of a known genetic syndrome. There was no association between the presence of VT and CMR measurements of left ventricular end diastolic volume or LV ejection fraction ( Table 2 ).
| Ventricular tachycardia (n = 8) | No ventricular tachycardia (n = 31) | p-value | |
|---|---|---|---|
| Patient Characteristics | |||
| Age at diagnosis (years) | 10.4 (3.9, 15.2) | 11.9 (5.1, 13.4) | 0.86 |
| Age at last follow up (years) | 16.7 (± 2.3) | 14.6 (± 5.1) | 0.1 |
| Follow up time (years) | 5.1 (1.4, 14.0) | 4.4 (1.8, 7.8) | 0.69 |
| Age at MRI (years) | 15.2 (± 2.8) | 12.6 (± 5.4) | 0.21 |
| Male (%) | 6 (75%) | 24 (77%) | 0.61 |
| Family history of hypertrophic cardiomyopathy (%) | 3 (37.5%) | 7 (22.6%) | 0.33 |
| Genetic syndrome (%) | 0 | 5 (16.1%) | 0.3 |
| CMR Measure | |||
| Left ventricular end-diastolic volume (ml/m 2 ) | 81.4 (± 22.7) | 83.5 (± 22.4) | 0.81 |
| Left ventricular ejection fraction (%) | 69.9 (± 4.7) | 68.1 (± 11.7) | 0.67 |
| Left ventricular mass indexed to height 2.7 (g/m 2.7 ) | 76.4 (± 40.5) | 50.9 (± 24.3) | 0.03 |
| Maximum thickness (cm) | 2.73 (± 1.36) | 1.73 (± 0.64) | 0.08 |
| Number of left ventricular segments with late gadolinium enhancement | 5 (0-7) | 0 (0-4) | 0.07 |
| Number of left ventricular segments with hypertrophy | 9.5 (7-10) | 8 (5-10) | 0.31 |
LGE assessment was performed in 35 patients, and image quality was sufficient in 33 patients. LGE was present in 17 patients (52%) and limited to midmyocardial involvement in all but one patient with transmural involvement. LGE evaluation was included in the CMR protocol in 7 of the 8 patients with VT. Figure 2 depicts the distribution of subjects with respect to the number of LV segments with LGE. LGE was present in 5 of these patients (71%). There was no association between the presence of LGE and age at diagnosis with HC, age at magnetic resonance imaging, gender, or the presence of family history of HC or a genetic syndrome ( Table 3 ). The midanteroseptal, midinferoseptal, and apical septal segments were the most commonly involved segments while in no patient were the basal anterior, basal inferolateral, basal anterolateral, or apical segments found to have LGE ( Figure 3 , Table 4 ). LGE in the apical septal (p = 0.03), basal inferoseptal (p <0.01), and basal inferior (p = 0.04) segments were specifically associated with VT ( Table 4 ).
| Late gadolinium enhancement (n = 17) | No Late gadolinium enhancement (n = 16) | p-value | |
|---|---|---|---|
| Patient characteristics | |||
| Age at diagnosis (years) | 12.0 (8.6, 15.4) | 12.0 (1.5, 13.8) | 0.53 |
| Age at last follow up (years) | 15.5 (±4.6) | 14.8 (±4.9) | 0.64 |
| Age at magnetic resonance imaging | 13.9 (±4.8) | 13.3 (±5.0) | 0.69 |
| Male (%) | 14 (82%) | 15 (94%) | 0.32 |
| Family history of hypertrophic cardiomyopathy (%) | 7 (41.2%) | 3 (19%) | 0.15 |
| Genetic syndrome (%) | 1 (6%) | 2 (13%) | 0.48 |
| Cardiac magnetic resonance imaging measure | |||
| Left ventricular mass indexed to height 2.7 (g/m 2.7 ) | 67.2 (±33.5) | 42.3 (±15.1) | 0.01 |
| Maximum thickness (cm) | 2.5 (±1.1) | 1.5 (±0.3) | < 0.01 |
| Number of left ventricular segments with hypertrophy | 10 (7-11.5) | 7 (5-9) | 0.02 |
| Left ventricular end-diastolic volume (ml/m 2 ) | 87.7 (±22.0) | 82.8 (±19.2) | 0.50 |
| Left ventricular ejection fraction (%) | 65.6 (±14.3) | 71.4 (±6.3) | 0.15 |
| LV Segment with LGE | Patients with LGE (N = 17) | Patients with LGE and VT (N = 7) | p-value ∗ |
|---|---|---|---|
| Mid Anteroseptal | 15 | 5 (71%) | 0.13 |
| Mid Inferoseptal | 13 | 4 (57%) | 0.26 |
| Apical Septal | 11 | 5 (71%) | 0.03 |
| Mid Anterolateral | 6 | 1 (14%) | 0.62 |
| Basal Inferoseptal | 5 | 4 (57%) | < 0.01 |
| Mid Anterior | 4 | 1 (14%) | 0.64 |
| Mid Inferior | 4 | 1 (14%) | 0.64 |
| Apical Anterior | 4 | 2 (29%) | 0.19 |
| Apical Lateral | 4 | 1 (14%) | 0.64 |
| Mid Inferolateral | 3 | 1 (14%) | 0.52 |
| Apical Inferior | 3 | 2 (29%) | 0.11 |
| Basal Anteroseptal | 2 | 1 (14%) | 0.38 |
| Basal Inferior | 2 | 2 (29%) | 0.04 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


