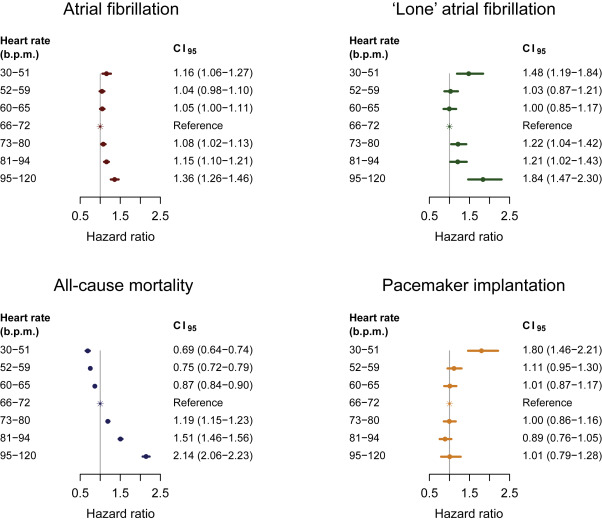
Heart rate (HR) at rest is a well-known marker of cardiovascular morbidity and mortality. Results on the association between HR and incident atrial fibrillation (AF) have, however, been conflicting. Using digital electrocardiograms from 281,451 primary care patients, we aimed to describe the association between HR at rest and the hazards of incident AF. Secondary end points were death from all causes and pacemaker implantation. Data on drug use, co-morbidity, and outcomes were collected from nationwide administrative health care registries. During a median follow-up time of 8.4 years, 15,666 subjects were observed to develop AF, of which 1,631 were lone AF. A HR at rest from 30 to 51 beats/min was associated with an adjusted hazard ratio of 1.16 (95% CI 1.06 to 1.27) for AF compared with the reference group (66 to 72 beats/min). From 72 beats/min and upward, the hazard ratio of AF increased in a dose-response manner, reaching an adjusted hazard ratio of 1.36 (95% CI 1.26 to 1.46) for HR between 95 and 120 beats/min. Both for low and high HR, the associations were accentuated for the outcome lone AF (adjusted hazard ratios of 1.48, 95% CI 1.19 to 1.84 and 1.84, 95% CI 1.47 to 2.30 for HR between 30 to 51 and 95 to 120 beats/min, respectively). For death from all causes, the hazard increased almost linearly with increasing HR. A HR at rest from 30 to 51 beats/min was associated with an adjusted hazard ratio of 1.80 (95% CI 1.46 to 2.21) for pacemaker implantation. In conclusion, a U-shaped association was found between HR at rest and incident AF, and this association was strongest for the outcome lone AF.
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia, affecting 1% to 2% of the general population. AF represents a major health care burden in terms of morbidity and mortality, primarily due to an increased risk of ischemic stroke and heart failure. Heart rate (HR) at rest has long been recognized as a marker of cardiovascular morbidity as well as all-cause and cardiovascular mortality. However, results on the association between HR at rest and incident AF have been conflicting. Using data on a large population of primary care patients referred for digital electrocardiographic (ECG) recording, we aimed to describe the association between HR at rest and incident AF, allowing for a nonlinear relation. Secondary end points were all-cause mortality and pacemaker implantation.
Methods
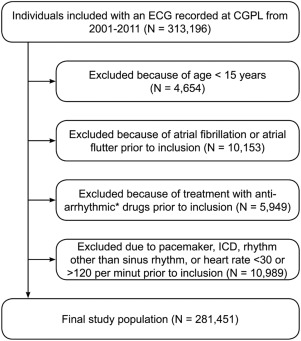
Most general practitioners in the greater region of Copenhagen, Denmark, refer their patients to the Copenhagen General Practitioners’ Laboratory (CGPL) for clinical tests, such as ECG recordings. The present study is part of the Copenhagen ECG study including all subjects who had an electrocardiogram recorded at the CGPL during 2001 to 2011, as described in details previously. For the present analysis, subjects were excluded as shown in Figure 1 .
All electrocardiograms obtained at CGPL were recorded on subjects at rest and in supine position. Recordings were digitally stored in the MUSE Cardiology Information System (GE Healthcare, Wauwatosa, Wisconsin), and processed using version 21 of the Marquette 12SL algorithm. With the use of this algorithm, we excluded electrocardiograms with rhythms different from sinus rhythm, delta waves, short PR intervals (<50 ms), second and third degree atrioventricular blocks, multiple premature ventricular complexes, multiple premature atrial complexes, junctional rhythms, and pace spikes. The algorithm derived the HR based on the number of sinus QRS complexes during a 10-second recording. For subjects with multiple electrocardiograms obtained, only the first was taken into account. However, to get an estimate of the intraindividual ECG-to-ECG variability of HR, we identified subjects with a second ECG recording 1 year ± 3 months (n = 18,676) and 8 years ± 1 year (n = 20,901) from the first recording (baseline electrocardiogram) and estimated the medians of the absolute differences in HR between the 2 electrocardiograms.
In Denmark, it is possible to follow subjects with respect to death, emigration, the use of prescription medicine and any hospital, outpatient clinic, or emergency room discharge diagnosis with the use of national health care registries and a unique personal identification number. In this way, we identified subjects with the following baseline characteristics: hypertension, heart failure, myocardial infarction, valvular heart disease, diabetes, hyperthyroidism, and treatment with HR-lowering medication (β blockers or calcium antagonists) at the day of ECG recording. Hypertension was defined as being present if a subject before inclusion was treated simultaneously with at least 2 kinds of antihypertensive drugs. Heart failure was defined as a discharge diagnosis of heart failure in combination with treatment with loop diuretics. Myocardial infarction and valvular heart disease were defined from discharge diagnosis, procedure, and operation codes. Diabetes and hyperthyroidism were defined from discharge diagnosis or in case of a purchase of prescription medication used for 1 of the 2 diseases. The primary event of interest was a hospital, outpatient clinic, or emergency room discharge diagnosis of AF or atrial flutter. Lone AF was defined as the occurrence of AF before the age of 65 years and in the absence of hypertension, heart failure, myocardial infarction, valvular heart disease, diabetes, and hyperthyroidism. A hospital, outpatient clinic, or emergency room discharge diagnosis of pacemaker implantation and death from all causes were secondary end points. Detailed information on the identification of covariates and clinical outcomes in the Danish registries has previously been provided.
Follow-up began on the day of the first ECG recording (index electrocardiogram) and ended in case of the event of interest, death, emigration, or on December 31, 2013, whichever came first. The median follow-up time was estimated with the reverse Kaplan–Meier method. Separate analyses were performed for outcomes AF, lone AF, pacemaker implantation, and death from all causes. Analyses of the outcome lone AF excluded patients with hypertension, heart failure, myocardial infarction, valvular heart disease, diabetes, hyperthyroidism, ECG criteria of left ventricular hypertrophy, and/or age >65 years at the date of index electrocardiogram (n = 117,387). Follow-up for the outcome lone AF was stopped at an event (diagnosis) of hypertension, heart failure, myocardial infarction, valvular heart disease, diabetes, or hyperthyroidism or surpassing 65 years of age. Cause-specific Cox regression was used to assess the association of HR, measured on the index electrocardiogram, with the hazard of AF, lone AF, pacemaker implantation, and death from all causes, respectively. All analyses were adjusted for conventional risk factors (see legend to Figure 2 ) that were obtained at the date of index electrocardiogram using a stratified Cox regression analysis. Age was categorized into 1-year intervals, and the baseline hazard rate was stratified according to age and all other risk factors. Thus, no proportional hazard assumption was made, except for the effect of HR. The latter was checked and accepted by means of “stopped Cox regression.” The population was divided into 7 categories based on HR (index electrocardiogram), with cutoffs at the 5th, 20th, 40th, 60th, 80th, and 95th percentiles. Hazard ratios with corresponding 95% CIs are reported using the middle HR category (40th to <60 percentile) as reference group. A 2-sided p value of <0.05 was considered statistically significant. All analyses were conducted with the use of R (R Foundation for Statistical Computing, Vienna, Austria [URL http://www.R-project.org/ ]).
According to Danish law, no approval from an ethics committee is needed in a registry-based study without any active participation from study subjects. The use of deidentified registry data was approved by the Danish Data Protection Agency (record number 2007-58-0015).
Results
The final study population consisted of 281,451 subjects with a median follow-up time of 8.4 years (interquartile range [IQR] 5.5 to 10.9 years). Baseline characteristics of the study population are presented in Table 1 . No clinically relevant gender-specific differences were present in any models.
| Characteristics | Overall 30–120 beats/min (N = 281451) | Percentiles of heart rate and associated interval limits | ||||||
|---|---|---|---|---|---|---|---|---|
| < 5th 30–51 beats/min (n = 12198) | 5th to < 20th 52–59 beats/min (n = 40260) | 20th to < 40th 60–65 beats/min (n = 51067) | 40th to < 60th 66–72 beats/min (n = 63336) | 60th to < 80th 73–80 beats/min (n = 54281) | 80th to < 95th 81–94 beats/min (n = 45257) | ≥ 95th 95–120 beats/min (n = 15052) | ||
| Age (years) | 55 (42 – 67) | 49 (37 – 61) | 52 (40 – 63) | 53 (41 – 65) | 55 (42 – 66) | 56 (42 – 68) | 58 (44 – 71) | 59 (45 – 71) |
| Women | 156397 (56%) | 4265 (35%) | 17973 (45%) | 27031 (53%) | 36919 (58%) | 33345 (61%) | 27913 (62%) | 8951 (59%) |
| Medical history | ||||||||
| Hypertension | 48152 (17%) | 1807 (15%) | 6089 (15%) | 8032 (16%) | 10347 (16%) | 9820 (18%) | 9021 (20%) | 3036 (20%) |
| Heart failure | 2250 (.8%) | 84 (.7%) | 236 (.6%) | 301 (.6%) | 444 (.7%) | 438 (.8%) | 522 (1%) | 225 (1%) |
| Myocardial infarction | 7773 (3%) | 458 (4%) | 1311 (3%) | 1430 (3%) | 1644 (3%) | 1353 (2%) | 1179 (3%) | 398 (3%) |
| Valvular heart disease | 1100 (.4%) | 49 (.4%) | 148 (.4%) | 187 (.4%) | 210 (.3%) | 228 (.4%) | 206 (.5%) | 72 (.5%) |
| Diabetes mellitus | 15101 (5%) | 344 (3%) | 1404 (3%) | 2111 (4%) | 3197 (5%) | 3337 (6%) | 3460 (8%) | 1248 (8%) |
| Hyperthyroidism | 3939 (1%) | 123 (1%) | 487 (1%) | 643 (1%) | 875 (1%) | 815 (2%) | 757 (2%) | 239 (2%) |
| Heart-rate lowering medication at the day of ECG recording ∗ | 32557 (12%) | 1425 (12%) | 4630 (12%) | 5790 (11%) | 6887 (11%) | 6295 (12%) | 5645 (12%) | 1885 (13%) |
| ECG variables | ||||||||
| PR interval (milliseconds) | 156 (142 – 172) | 162 (148 – 180) | 158 (144 – 176) | 158 (144 – 174) | 156 (142 – 172) | 154 (142 – 170) | 152 (140 – 168) | 154 (140 – 172) |
| Heart rate (beats/min) | 70 (62 – 79) | 49 (46 – 50) | 56 (54 – 58) | 63 (61 – 64) | 69 (67 – 71) | 76 (74 – 78) | 86 (83 – 89) | 100 (97 – 105) |
| Left ventricular hypertrophy † | 12388 (4%) | 1059 (9%) | 2236 (6%) | 2307 (5%) | 2395 (4%) | 1970 (4%) | 1736 (4%) | 685 (5%) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


