The association between the anatomic characteristics obtained by omni-plane transesophageal echocardiography (TEE) and stroke recurrence in patients with cryptogenic stroke and patent foramen ovale (PFO) remains unclear. In the present longitudinal follow-up study, we sought to investigate whether PFO findings assessed by TEE can predict stroke recurrence. Of the 1,014 consecutive patients with acute ischemic stroke referred for TEE, 184 (mean ± SD age, 51 ± 14 years) were classified as having cryptogenic stroke with PFO, and follow-up data were available for 181 patients. During follow-up (median 3.5 years), 14 patients (7.7%) experienced stroke recurrence. Multivariate analysis showed that atrial septal aneurysm or hypermobility of the atrial septum (hazard ratio 6.04, 95% confidence interval 1.84 to 19.86, p = 0.003) and PFO size (hazard ratio 3.00, 95% confidence interval 1.96 to 4.60, p <0.0001) were independent predictors of stroke recurrence. The optimal cutoff value of PFO to predict stroke recurrence within 3 years was 3.0 mm (95% confidence interval 2.1 to 3.7 mm, area under the curve 0.889, p <0.001) with a sensitivity and specificity of 90.0% and 79.4%, respectively. Using this cutoff, the 3-year stroke recurrence-free survival rates differed significantly (98.9 ± 1.1% vs 71.5 ± 16.2%, p <0.001). In conclusion, our data suggest that risk stratification might be possible using the findings from TEE. The prophylactic benefit of PFO closure from these findings needs additional investigation.
The appropriate treatment strategy for secondary stroke prevention in patients with cryptogenic stroke and patent foramen ovale (PFO) remains challenging. The clinical and anatomic variables reported to be risk factors associated with stroke recurrence include older age, large PFO, large right-to-left shunting, and combined atrial septal aneurysm (ASA). However, these factors were not confirmed by other studies. Most clinical observations have been from the results of cross-sectional and case-control studies, which have flaws, including poorly defined control groups, case selection according to age, and heterogeneous imaging techniques, including transthoracic and old mono- and biplane transesophageal echocardiography (TEE). To overcome these limitations, the present longitudinal follow-up study of patients with cryptogenic stroke and PFO explored the relation between the anatomic features of atrial septal abnormalities, evaluated by the standard omni-plane TEE, and stroke recurrence, regardless of patient age. The aim of the present study was to determine whether risk stratification to predict stroke recurrence was possible using the findings from TEE, which could help to establish guidelines for secondary prevention.
Methods
All patients with acute ischemic stroke referred to the Stroke Center, Asan Medical Center from January 2000 to April 2007 were screened for enrollment. A standardized protocol to determine the definite causes of ischemic stroke has been used at our institution since 1995. For the diagnosis of cryptogenic stroke, patients who were considered to have definite causes of stroke were excluded : (1) large-artery atherosclerosis (defined as stenosis ≥50% or occlusion of the corresponding artery); (2) lacunar stroke (defined as a small, deep infarct ≤20 mm in diameter without arterial or cardiac sources of embolism; (3) cardioembolic causes, such as atrial fibrillation, recent (within 4 months) myocardial infarction, dilated cardiomyopathy, rheumatic mitral stenosis, mitral or aortic vegetations or prostheses, left atrial or left ventricular thrombus or tumor, akinetic left ventricular segment, spontaneous echocardiographic contrast of the left atrium, and complex atheroma of the aortic arch; and (4) other definite causes of stroke, such as nonatherosclerosis arteriopathies (e.g., dissection), coagulopathies, and hematologic or systemic disorders (e.g., the antiphospholipid-antibody syndrome).
A strictly predefined protocol, performed by experienced echocardiographic specialists (JKS, DHK, and JMS), was used to assess for PFO and ASA at rest and during the Valsalva maneuver using 5-MHz omni-plane transesophageal echocardiographic transducers and hand-agitated saline. After careful observation of the interatrial septum using 2-dimensional echocardiography, color Doppler mapping and M-mode tracing of the septum with the Valsalva maneuver ( Figure 1 A–C), ≥3 intravenous contrast injections (5 ml of hand-agitated saline) were administered through the right antecubital vein at rest and with the Valsalva maneuver, followed by deep inspiration ( Figure 1 D–F). PFO was defined as passage of contrast (≥3 microbubbles) from the right to left atrium through the gap, within 3 cardiac cycles after complete opacification of the right atrium. All diagnostic discrepancies were resolved by a second reviewer unaware of the patient details.
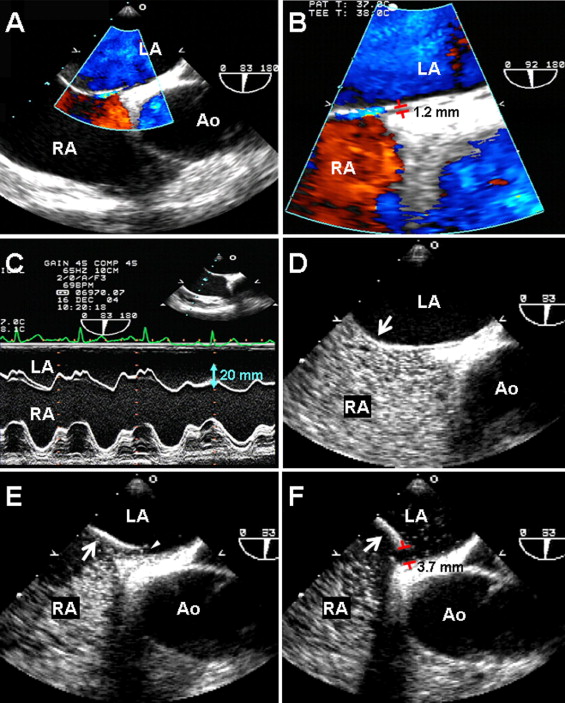
Digitally, stored transesophageal echocardiographic images were reviewed and analyzed by an investigator (JYL) who was unaware of the clinical outcomes. Using calipers, the PFO size was measured as the maximum separation of the septum primum from the septum secundum ( Figure 1 F). The maximum number of the microbubbles in the left atrium within 3 cardiac cycles after complete opacification of the right atrium was also calculated. The PFO size and shunt quantification were measured ≥3 times, and the mean value of each was calculated. We classified grade 1 as minimal (≤5 bubbles), grade 2 as moderate (6 to 20 bubbles), and grade 3 as severe (>20 bubbles). ASA or hypermobility was defined as ≥10 mm of phasic septal excursion either into the atrium or a sum total excursion of >15 mm during the cardiorespiratory cycle, with a base of ≥15 mm. The maximum length of the eustachian valve was also measured in the digital images.
Except for 3 patients who refused additional procedures or medication, the treatment strategy for secondary prevention in patients with cryptogenic stroke and PFO was determined at the discretion of the attending stroke neurologist and was determined from the stroke pattern, patient age, co-morbidities, and cardiovascular risk factors. Patients assigned to medical treatment were treated with either a vitamin K antagonist or antiplatelet therapy. Warfarin was adjusted to a target international normalized ratio of 2.0 to 3.0. In some patients, PFO closure was performed percutaneously with the patient under general anesthesia and with transesophageal echocardiographic guidance.
The clinical data from patients with cryptogenic stroke and PFO were regularly recorded by neurologists during the regular medical visits. Patients who failed to make a regular visit were interviewed by telephone to determine whether they had experienced recurrent ischemic stroke, the primary end point of the present study. Recurrence of ischemic stroke was confirmed by a new lesion on computed tomography or magnetic resonance imaging. The clinical follow-up data obtained thorough the end of August 2008 were used in the final analysis. The institutional review board of Asan Medical Center approved the present retrospective study.
The primary end point of the study was stroke recurrence. The data are presented as the mean or median ± SD or median and range, as appropriate. Between group comparisons of the baseline and transesophageal echocardiographic variables were done using Student’s t test, the Wilcoxon rank sum test, analysis of variance, or the Kruskal-Wallis test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables, as appropriate. Unadjusted cumulative event rates were estimated using the Kaplan-Meier method and were compared using the log-rank test. Independent predictors of recurrence were analyzed using the Cox proportional hazard model. For recurrence, owing to the infrequent occurrence of events, the predictive value of univariate findings was subsequently tested with a bootstrap resampling procedure in which multivariate Cox regression analysis with a backward elimination process was repeated for 1,000 bootstrap resampling. The relative frequency of selection of the bootstrap resampling >50% was used as the criterion for inclusion of predictors in the final multivariate model. The proportional hazards assumption was confirmed by testing of partial (Schoenfeld) residuals, and no relevant violations were found. To assess the cutoff value of PFO size for predicting recurrence within 3 years, the receiver operating characteristic curve was used. We excluded censoring data within 3 years, and recurrence after 3 years was censored. The optimal cutoff value was defined as the value with the maximum sum of sensitivity and specificity. The reported 95% confidence intervals for the cutoff value, area under the curve, sensitivity, and specificity were obtained using the bootstrap with percentile method (220 replicate).
The intra- and interobserver variability of PFO size measurement were evaluated in 50 randomly selected patients. The correlation coefficient of the 2 measurements and the coefficient of variation, defined as the SD of the measured difference as a percentage of the average of the 2 series, were calculated. The intraobserver correlation coefficient and coefficient of variation was 0.960 (p <0.001) and 9.9%, respectively, and the interobserver correlation coefficient and the coefficient of variation was 0.922 (p <0.001) and 18.8%, respectively.
All p values are 2-sided, and p <0.05 was considered statistically significant. Statistical Analysis System software, version 9.1 (SAS Institute, Cary, North Carolina) and R programming language with DiagnosisMed library were used for statistical analysis.
Results
During the study period, 4,543 patients were admitted because of acute (<7 days after onset) ischemic stroke. Of these, 1,014 patients (22.3%) were referred for echocardiography to determine the potential cardiac source of embolism. PFOs were identified in 229 patients (22.6%); 45 patients had coexisting high-risk cardioembolic sources of stroke. Thus, the remaining 184 were classified as having cryptogenic stroke and PFO and were the subjects of the present study. Their mean ± SD age was 51 ± 14 years, and 135 (73.4%) were men. The mean ± SD PFO diameter was 2.0 ± 1.2 mm, and no significant change was found in PFO size according to age (p = 0.586). However, women had larger PFOs than men (2.3 ± 1.4 vs 1.9 ± 1.1 mm, p = 0.041).
Of the 184 patients, 22 underwent percutaneous closure of the PFO using an Amplatzer device (18 mm in 10 and 25 mm in 11 patients) or a CardioSEAL (17 mm in 1 patient; NMT Medical, Boston, Massachusetts). TEE immediately after the procedure confirmed successful closure in all 22 patients, and no serious complications occurred. Follow-up echocardiography (TEE and transthoracic echocardiography in 5 and 14 patients, respectively) was performed in 19 patients within 2 months after the procedure. Only 1 patient (4.5%) showed a minimal grade 1 residual right to left shunt with contrast echocardiography using hand-agitated saline. No patient had a thrombus on the device. During clinical follow-up of 2.9 ± 4.0 years (median 2.5), no recurrence developed of any embolic stroke.
Of the 99 patients who received antiplatelet medication (antiplatelet group), acetylsalicylic acid (100 mg/day) was the main regimen for 78 patients, either alone (n = 14) or combined with other antiplatelet drugs, such as clopidogrel (75 mg/day, n = 49) and cilostazol (100 mg twice daily, n = 11). The remaining patients received monotherapy with clopidogrel (n = 10) or cilostazol (n = 6) or a combination of these 2 medications. Of the 60 patients who received warfarin (anticoagulation group), 27 continued taking this drug, and 33 changed to antiplatelet drugs at 6 to 12 months after the initial ischemic stroke. No significant major bleeding episodes causing interruption of therapy occurred but minor bleeding episodes (e.g., epistaxis, bruising, ecchymosis, and petechiae) were observed in 5 patients (8.3%) in the anticoagulation group. Table 1 lists the baseline and echocardiographic variables according to treatment group in the patients with cryptogenic stroke and PFO.
| Variable | Antiplatelet Group (n = 99) | Anticoagulation Group (n = 60) | Surgical Closure Group (n = 22) | p Value |
|---|---|---|---|---|
| Demographic data | ||||
| Age (years) | 53 ± 13 | 53 ± 13 | 41 ± 12 ⁎ † | 0.001 |
| Men | 69 (70%) | 48 (80%) | 15 (63%) | 0.34 |
| Hypertension | 54 (55%) | 28 (47%) | 2 (9%) ⁎ † | 0.001 |
| Diabetes mellitus | 17 (17%) | 10 (17%) | 0 (0%) ⁎ | 0.03 |
| Cholesterol >240 mg/dl | 25 (25%) | 14 (24%) | 6 (27%) | 0.85 |
| Smoker | 35 (35%) | 23 (38%) | 8 (36%) | 0.87 |
| Family history of stroke | 5 (5%) | 2 (3%) | 10 (5%) | 0.85 |
| Echocardiographic data | ||||
| Left to right shunt | 27 (27%) | 24 (40%) | 16 (69%) ⁎ † | <0.001 |
| Right-to-left shunt at rest | 8 (8%) | 4 (7%) | 1 (5%) | 0.13 |
| Shunt grade 3 | 14 (14%) | 8 (13%) | 11 (50%) ⁎ † | <0.001 |
| Atrial septal aneurysm or hypermobility | 11 (11%) | 7 (12%) | 1 (5%) | 0.65 |
| Patent foramen ovale size (mm) | 2.0 ± 1.2 | 1.9 ± 1.1 | 2.4 ± 1.2 | 0.10 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


