Although inflammation is involved in the pathogenesis of acute coronary syndromes, the extent of inflammation is not routinely assessed, and its prognostic implications in patients with non–ST-segment elevation acute coronary syndrome have not been investigated in depth. We analyzed the prognostic implications of an elevated white blood cell count (WBCc) in patients with moderate and high-risk non–ST-segment elevation acute coronary syndrome undergoing an early invasive strategy in the large-scale Acute Catheterization and Urgent Intervention Triage StrategY trial. The WBCc at admission was available for 13,678 of 13,819 patients (98.9%). The patients in the upper tertile of the WBCc had an increased risk of 30-day major bleeding, 1-year mortality, and definite/probable stent thrombosis compared to those in the mid or lower tertiles. On multivariate analysis, the WBCc was an independent predictor of 30-day major bleeding and 1-year cardiac, noncardiac, and all-cause mortality. The association between the WBCc and cardiac mortality was present in multiple prespecified subgroups, with no significant interaction between the WBCc and age, gender, diabetes, smoking, renal dysfunction, elevated baseline biomarkers, antithrombotic therapy, revascularization, and Thrombolysis In Myocardial Infarction risk score. The WBCc remained an independent predictor of mortality after adjusting for bleeding, C-reactive protein level, and angiographic variables, including left ventricular ejection fraction, Thrombolysis In Myocardial Infarction flow, and number of diseased vessels. The WBCc significantly improved the prognostic accuracy of the Thrombolysis In Myocardial Infarction risk score, with a net reclassification improvement of 11% (p <0.0001). In conclusion, in patients with moderate- and high-risk non–ST-segment elevation acute coronary syndrome, an elevated admission WBCc was an independent predictor of 30-day major bleeding, and 1-year cardiac, noncardiac, and all-cause mortality.
Studies have suggested that inflammation plays a pivotal role in the pathogenesis of acute coronary syndromes (ACS). The prognostic implications of inflammation in patients with non–ST-segment elevation ACS (NSTE-ACS) have not been investigated in depth. The white blood cell count (WBCc) is an attractive marker for potential risk stratification of patients with NSTE-ACS because of its low cost and routine assessment at hospital admission. Previous studies have found a correlation between the WBCc and the severity of systemic inflammation, and an elevated WBCc has been suggested as a predictor of short-term mortality in patients with NSTE-ACS. However, whether this association is influenced by coronary revascularization, is co-linear with C-reactive protein (CRP), or is driven by cardiac or noncardiac mortality remains undetermined. Moreover, the relation between the WBCc and major bleeding and stent thrombosis has never been investigated in this subset of patients. We, therefore, examined the relation between the admission WBCc and clinical outcomes in moderate- and high-risk patients with NSTE-ACS undergoing an early invasive strategy in the large-scale, multicenter, prospective, randomized Acute Catheterization and Urgent Intervention Triage StrategY (ACUITY) trial.
Methods
The ACUITY trial design has been previously reported in detail. In brief, the ACUITY trial was a multicenter, prospective randomized trial of patients with moderate- and high-risk NSTE-ACS who were treated with an early invasive strategy. Patients were randomly assigned before coronary angiography to heparin (unfractionated or low-molecular-weight) plus a glycoprotein IIb/IIIa inhibitor, bivalirudin plus a glycoprotein IIb/IIIa inhibitor, or bivalirudin monotherapy with provisional glycoprotein IIb/IIIa inhibitor use. Angiography was performed in all patients within 72 hours of randomization. Depending on the coronary anatomy, the patients then received percutaneous coronary intervention, coronary artery bypass grafting, or medical therapy. In patients undergoing percutaneous coronary intervention, the choice of either bare metal or drug-eluting stents was at the operator’s discretion. Dual antiplatelet therapy with aspirin and clopidogrel was strongly recommended for ≥1 year. The WBCc at admission was required for all patients by protocol and was determined using standard laboratory assays at the local enrolling centers. All major adverse events were adjudicated by an independent clinical events committee who was unaware of the treatment assignment and the WBCc.
The primary objective of the present study was to investigate the association between the WBCc and 1-year all-cause mortality. The secondary end points of interest included the 1-year rates of cardiac and noncardiac mortality, myocardial infarction (MI), target vessel revascularization, and definite/probable stent thrombosis defined according to the Academic Research Consortium definition. We also assessed noncoronary artery bypass grafting–related major bleeding at 30 days (the latest this end point was adjudicated). Renal dysfunction was defined as a calculated creatinine clearance using the Cockcroft-Gault equation of <60 ml/min. The extent of coronary artery disease was defined as the sum of the lengths of all lesions in the coronary tree with >30% diameter stenosis in vessels with a reference diameter of ≥1.5 mm determined using quantitative coronary angiography as previously described.
The continuous data are presented as the mean ± SD and were compared using analysis of variance. Categorical variables are summarized as percentages and were compared using the chi-square test or Fisher’s exact test. All outcomes were determined using the Kaplan-Meier time-to-event method and compared using the log-rank test. Analyses were stratified according to tertiles of the baseline WBCc. Stepwise Cox multivariate regression analyses were performed with entry and exit criteria set at p ≤0.1 to assess the association between the WBCc and the clinical end points, after confirmation that the proportional hazards assumption was met. The variables included in the model were WBCc (as a continuous variable), age, gender, diabetes mellitus, smoking, hypertension, hypercholesterolemia, baseline creatine phosphokinase value, ST-segment deviation ≥1 mm, previous MI, previous percutaneous coronary intervention, previous coronary artery bypass grafting, renal dysfunction, therapy with glycoprotein IIb/IIIa inhibitor, and treatment type (percutaneous coronary intervention vs coronary artery bypass grafting vs medical therapy).
To address the potential effect of the angiographic variables, multivariate analyses were also performed in the subgroup of patients included in the formal angiographic substudy of the ACUITY trials. The variables included in these models were WBCc (as a continuous variable), age, male gender, diabetes, smoking, previous MI, ST-segment deviation, renal insufficiency, creatine phosphokinase level, treatment type (percutaneous coronary intervention vs coronary artery bypass grafting vs medical therapy), number of lesions, extent of coronary artery disease, number of vessel disease, jeopardy score, and left ventricular ejection fraction. The prognostic accuracy of the WBCc to predict 1-year all-cause mortality, cardiac mortality, noncardiac mortality, and noncoronary artery bypass grafting–related major bleeding was assessed using Harrell’s concordance index. To further investigate whether the WBCc improved the prognostic accuracy of the Thrombolysis In Myocardial Infarction risk score to predict 1-year mortality, the net reclassification improvement and integrated discrimination improvement were determined for the upper tertile versus the mid plus lower tertiles of the admission WBCc. Statistical analyses were performed using SAS, version 9.1 (SAS Institute, Cary, North Carolina). p Values <0.05 were considered statistically significant.
Results
Of the 13,819 patients enrolled in the ACUITY trial, 13,678 (98.9%) had a baseline WBCc available and represented the present study cohort. The clinical characteristics of the patients stratified by WBCc tertiles are listed in Table 1 . The patients in the greater WBCc tertile were younger and were more likely to be smokers and to have elevated cardiac biomarkers or ST-segment deviation ≥1 mm but were less likely to have had previous MI, percutaneous coronary intervention, or coronary artery bypass grafting compared to patients in the mid or lower tertiles.
| Variable | Tertile I (≤7,000/mm 3 ; n = 4,561) | Tertile II (>7,000/mm 3 and ≤9,200/mm 3 ; n = 4,646) | Tertile III (>9,200/mm 3 ; n = 4,471) | p Value |
|---|---|---|---|---|
| Age (yrs) | 64.0 ± 11.3 | 62.7 ± 11.4 | 61.0 ± 12.1 | <0.0001 |
| Male gender | 69.8% (3,182/4,561) | 70.8% (3,290/4,646) | 69.2% (3,096/4,471) | 0.25 |
| Hypertension | 70.5% (3,206/4,546) | 67.4% (3,119/4,631) | 62.8% (2,795/4,448) | <0.0001 |
| Diabetes mellitus | 28.9% (1,310/4,535) | 27.3% (1,262/4,619) | 27.9% (1,236/4,434) | 0.24 |
| Insulin-treated diabetes mellitus | 8.2% (370/4,535) | 8.6% (396/4,619) | 9.2% (410/4,434) | 0.18 |
| Hypercholesterolemia | 63.9% (286/4,488) | 57.2% (2,622/4,585) | 50.0 (2,175/4,347) | <0.0001 |
| Current smoker | 16.9% (757/4,484) | 28.1% (1,282/4,570) | 42.6% (1,870/4,387) | <0.0001 |
| Previous myocardial infarction | 34.9% (1,557/4,461) | 31.1% (1,413/4,537) | 27.5% (1,202/4,368) | <0.0001 |
| Previous percutaneous coronary intervention | 47.7% (2,155/4,520) | 39.2% (1,806/4,608) | 29.2% (1,296/4,433) | <0.0001 |
| Previous coronary artery bypass grafting | 21.6% (985/4,554) | 18.7% (866/4,639) | 13.3% (594/4,460) | <0.0001 |
| Renal dysfunction ∗ | 19.9% (850/4,266) | 18.9% (827/4,377) | 18.5% (782/4,230) | 0.22 |
| Left ventricular ejection fraction | 65.3% ± 12.0% (1,672) | 64.0% ± 11.9% (1,497) | 63.1% ± 12.6% (1,357) | <0.0001 |
| Baseline cardiac biomarker elevation | 46.5% (1,926/4,141) | 58.8% (2,518/4,283) | 72.9% (3,068/4,207) | <0.0001 |
| Baseline troponin elevation | 44.6% (1,699/3,811) | 57.8% (2,310/3,998) | 71.8% (2,869/3,995) | <0.0001 |
| ST-segment deviation ≥1 mm | 28.7% (1,307/4,561) | 35.0% (1,624/4,645) | 41.6% (1,860/4,467) | <0.0001 |
| Baseline cardiac biomarker elevation or ST-segment deviation ≥1 mm | 61.1% (2,609/4,271) | 72.5% (3,187/4,396) | 83.4% (3,607/4,323) | <0.0001 |
| Thrombolysis In Myocardial Infarction risk score | ||||
| Low (0–2) | 13.6% (546/4,001) | 15.8% (653/4,140) | 17.8% (709/3,988) | <0.0001 |
| Intermediate (3–4) | 55.3% (2,211/4,001) | 55.3% (2,288/4,140) | 53.1% (2,118/3,988) | 0.08 |
| High (5–7) | 31.1% (1,244/4,001) | 29.0% (1,199/4,140) | 29.1% (1,161/3,988) | 0.06 |
| Treatment strategy | ||||
| Percutaneous coronary intervention | 53.8% (2,453/4,561) | 56.1% (2,607/4,646) | 59.8% (2,672/4,471) | <0.0001 |
| Coronary artery bypass grafting | 10.2% (467/4,561) | 11.5% (533/4,646) | 11.9% (530/4,471) | 0.04 |
| Medical therapy | 36.0% (1,641/4,561) | 32.4% (1,506/4,646) | 28.4% (1,269/4,471) | <0.0001 |
| Antithrombotic medication after randomization | ||||
| Bivalirudin | 64.5% (2,941/4,561) | 65.9% (3,064/4,646) | 63.3% (2,831/4,471) | 0.03 |
| Unfractionated heparin | 17.3% (787/4,561) | 16.7% (778/4,646) | 17.1% (766/4,471) | 0.79 |
| Enoxaparin | 15.9% (727/4,561) | 15.6% (724/4,646) | 16.8% (753/4,471) | 0.24 |
| Glycoprotein IIb/IIIa inhibitors | 36.2% (1,652/4,561) | 37.4% (1,737/4,646) | 40.4% (1,808/4,471) | 0.0001 |
∗ Defined as calculated creatinine clearance rate of <60 ml/min using Cockcroft-Gault equation.
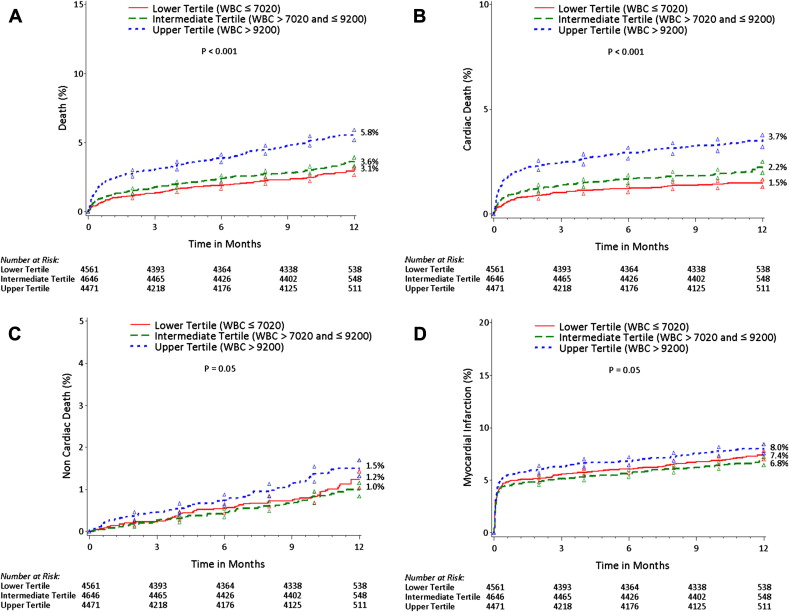
The 1-year follow-up rate was 96.2%, 96.3%, and 95.8% in the lower, mid, and upper tertile, respectively. As listed in Table E1 , the all-cause mortality during the first 30 days was greater in the patients in the upper tertile than in those in the intermediate and lower tertiles, driven by the significantly greater cardiac mortality. The noncoronary artery bypass grafting–related major bleeding and definite/probable stent thrombosis rates were also greater in the upper WBCc tertile. In contrast, the rates of MI and target vessel revascularization did not significantly vary according to the WBCc, although a trend toward an increased risk of MI was apparent for the upper WBCc tertile. At 1 year of follow-up ( Table E1 and Figure 1 ), the all-cause mortality remained greater for patients in the upper WBCc tertile, because of both greater cardiac ( Figure 1 ) and noncardiac ( Figure 1 ) mortality. Again, no significant differences were apparent in the rates of target vessel revascularization, although a borderline statistically significant difference was apparent for MI with a greater WBCc ( Figure 1 ). Moreover, the rate of definite/probable stent thrombosis remained significantly increased in patients in the upper tertile compared to those in the mid and lower tertile ( Table E1 ). As shown in Figure 2 , WBCc was associated with an increased risk of all-cause death and cardiac death, both within the first 30 days after the index hospitalization and within 30 days to 1 year. A similar trend was also observed for noncardiac mortality.
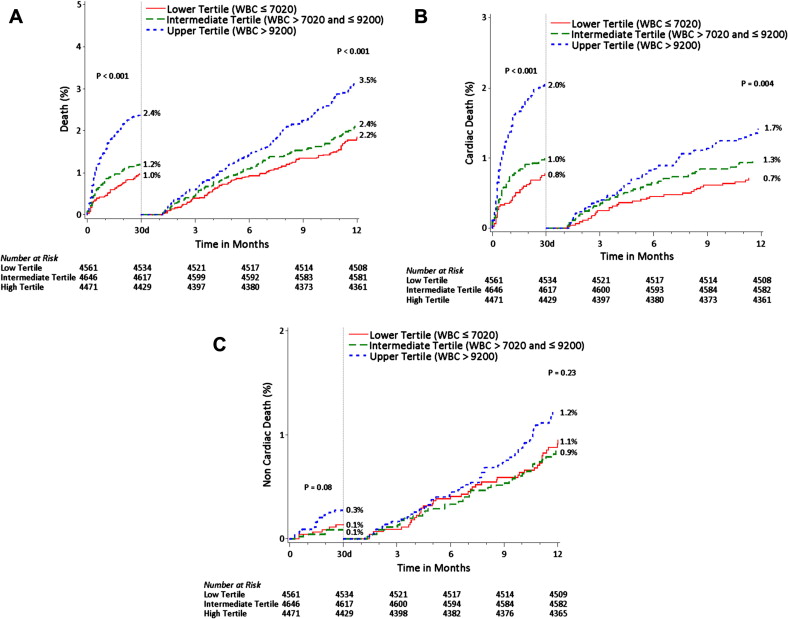
After adjusting for confounders, the WBCc was an independent predictor of 1-year all-cause mortality, cardiac mortality, and noncardiac mortality ( Table 2 ). Moreover, the WBCc was also an independent predictor of 30-day noncoronary artery bypass grafting–related major bleeding. The prognostic association between the WBCc and 1-year mortality also remained statistically significant after adjusting for noncoronary artery bypass grafting–related major bleeding (hazard ratio [HR] for WBCc 1.09, 95% confidence interval [CI] 1.07 to 1.11; HR for bleeding 3.13, 95% CI 2.16 to 4.55), with no interactions present between the WBCc and bleeding on the occurrence of 1-year mortality. In addition, the WBCc was an independent predictor of 1-year mortality in patients in whom major bleeding did not occur (HR 2.00, 95% CI 1.60 to 2.50; p <0.0001).
| HR (95% CI) | p Value | |
|---|---|---|
| All-cause mortality | ||
| White blood cell count (per 1,000-U increment) | 1.09 (1.08–1.11) | <0.0001 |
| Age (per 10-yr increments) | 1.07 (1.06–1.09) | <0.0001 |
| Male gender | 1.53 (1.24–1.90) | <0.0001 |
| Diabetes mellitus | 1.89 (1.56–2.29) | <0.0001 |
| Current cigarette smoking | 1.50 (1.18–1.92) | 0.001 |
| Previous myocardial infarction | 1.27 (1.04–1.55) | 0.02 |
| ST-segment deviation | 1.67 (1.38–2.02) | <0.0001 |
| Renal insufficiency | 1.45 (1.16–1.82) | 0.001 |
| Percutaneous coronary intervention vs medical therapy | 0.67 (0.55–0.83) | 0.0003 |
| Cardiac mortality | ||
| White blood cell count (per 1,000-U increment) | 1.10 (1.07–1.12) | <0.0001 |
| Age (per 10-yr increments) | 1.06 (1.04–1.08) | <0.0001 |
| Male gender | 1.46 (1.08–1.96) | 0.01 |
| Diabetes mellitus | 2.00 (1.53–2.61) | <0.0001 |
| Previous myocardial infarction | 1.47 (1.11–1.94) | 0.007 |
| ST-segment deviation | 2.29 (1.75–3.00) | <0.0001 |
| Renal insufficiency | 1.46 (1.07–1.99) | <0.0001 |
| Hypertension | 1.44 (1.03–2.02) | 0.03 |
| Creatine phosphokinase (per 1,000 U/L increment) | 1.28 (1.08–1.50) | 0.004 |
| Coronary artery bypass grafting vs medical therapy | 2.05 (1.41–2.98) | 0.0002 |
| Noncardiac mortality | ||
| White blood cell count (per 1,000-U increment) | 1.09 (1.06–1.12) | <0.0001 |
| Age (per 10-yr increments) | 1.09 (1.07–1.11) | <0.0001 |
| Male gender | 1.58 (1.09–2.28) | 0.01 |
| Current cigarette smoking | 1.85 (1.23–2.77) | 0.003 |
| Percutaneous coronary intervention vs medical therapy | 0.66 (0.46–0.93) | 0.02 |
| Noncoronary artery bypass graft–related major bleeding | ||
| White blood cell count (per 1,000-U increment) | 1.05 (1.03–1.07) | <0.0001 |
| Age (per 10-yr increments) | 1.03 (1.02–1.03) | <0.0001 |
| Male gender | 0.52 (0.44–0.61) | <0.0001 |
| Diabetes mellitus | 1.31 (1.10–1.55) | 0.002 |
| Renal insufficiency | 1.24 (1.05–1.47) | <0.0001 |
| Previous percutaneous coronary intervention | 0.75 (0.63–0.90) | 0.002 |
| Glycoprotein IIb/IIIa inhibitors | 2.42 (2.05–2.85) | <0.0001 |
| ST-segment deviation | 1.24 (1.05–1.47) | 0.01 |
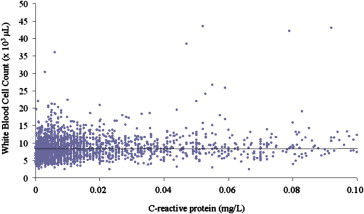
Among the subgroup of patients in whom the CRP levels were determined (n = 2,972), the WBCc remained an independent predictor of 1-year mortality after incorporation of CRP in the multivariate model (adjusted HR for WBCc 2.07, 95% CI 1.37 to 3.12, p = 0.0005; adjusted HR for CRP 1.33, 95% CI 1.10 to 1.61, p = 0.003). Only a weak correlation was apparent between the WBCc and CRP levels (r = 0.08, p <0.01; Figure 3 ). Moreover, as shown in Figure 4 , an elevated WBCc was associated with an increased risk of mortality in both patients with high CRP levels (CRP greater than the median [0.6 mg/dl]) and those with low CRP levels (CRP ≤0.6 mg/dl).