Assessment of the Thoracic Aorta
Steven Konstadt
This chapter will cover three main topics. First, it will describe the echocardiographic approach to the examination of the aorta. The anatomic proximity of the esophagus and aorta make it possible for an echocardiographer to interrogate nearly the entire thoracic aorta with the exception of the distal ascending and proximal arch portions. Then it will define the normal anatomy of the aorta, and finally it will discuss the pathologic conditions of the thoracic aorta.
The three pathologic conditions to be addressed are aortic dissection, aortic aneurysm, and aortic atherosclerosis. Acute dissection of the ascending aorta is a true medical emergency, often necessitating immediate surgical repair. The key to instituting appropriate therapy in acute dissection of the ascending aorta, therefore, is an accurate and rapid diagnosis and anatomical assessment of the aorta. Aortic aneurysm is the most common diagnosis of patients having elective surgery of the aorta. Though there is no universally accepted definition of an aneurysm, it is well accepted that aneurysms are dangerous and need to be treated. Neurologic dysfunction after cardiopulmonary bypass remains a major source of the morbidity and mortality associated with cardiac surgical procedures. Because atheroembolic phenomena are a predominant cause of adverse neurological outcomes in this patient population, atheromatous disease of the thoracic aorta is a significant risk factor for neurologic injury after cardiopulmonary bypass. Echocardiography may help to identify the patients at risk, and may provide risk reduction strategies.
ECHOCARDIOGRAPHIC APPROACH (TEE VIEWS, EPIAORTIC SCANNING)
In the pre- and postoperative periods, the thoracic aorta can be interrogated by transesophageal echocardiography. Additionally, in some patients, transthoracic images may be obtainable. In the intraoperative period after sternotomy, the thoracic aorta can be interrogated by both transesophageal and epiaortic techniques.
Transthoracic images of the ascending aorta may be obtained from the sternal notch by directing the probe towards the aortic valve using a standard transthoracic probe (Fig. 17.1). One limitation of this approach is the footprint of the probe and the size of the sternal notch window. Another problem with this approach is the relatively poor image quality compared to the other echocardiographic imaging options.
The Society of Cardiovascular Anesthesiologists and the American Society of Echocardiography have defined six views to interrogate the thoracic aorta by transesophageal echocardiography. However, it must be realized that these views are not single images to obtain, but rather six imaging planes to be used to evaluate the aorta. In most of the views, the probe must be manipulated to obtain multiple images in that orientation. The six imaging planes are
1. Midesophageal (ME) ascending aorta SAX (short axis)
2. ME ascending aorta LAX (long axis)
3. Upper esophageal (UE) aorta arch
4. UE aorta arch LAX
5. Descending aorta SAX
6. Descending aorta LAX
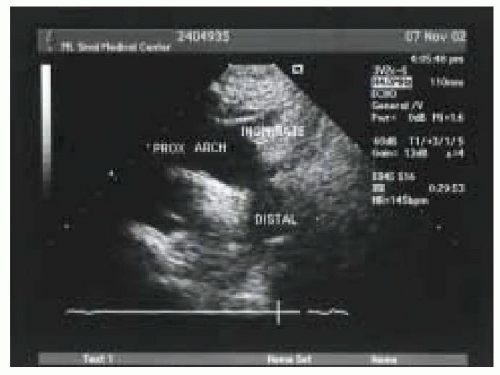
The ME ascending aorta SAX view is obtained in the 0-degree imaging plane with the probe at approximately 25 cm from the lips (Fig. 17.2). Frequently, anteflexion of the tip is needed to improve probe contact and therefore image quality. Though this view is called a midesophageal view, it is obtained closer to the lips than the “ME” views of the mitral valve, and therefore could be considered a UE view. In this view, a cross section of the ascending view of the aorta is obtained. This view is useful for qualitatively assessing the aortic anatomy and measuring the overall diameter of the aorta and the wall thickness. It is also useful for the assessment of the superior vena cava and the detection of pericardial fluid. Visualization of the main pulmonary artery and right pulmonary artery allows verification of proper pulmonary artery placement.
The ME ascending LAX view is obtained by rotating the imaging plane to between 90 and 110 degrees (Fig. 17.3). This view is useful for defining the wall thickness and tissue characteristics, aortic contour, aortic dimensions, and blood flow patterns in the ascending aorta. With respect to wall dimensions, this view is very useful to determine the relative diameter of the ascending aorta along its course. Unfortunately, because of the interposition of the airway between the esophagus and aorta only about 80% of the ascending aorta is visualized and in some patients more than 40% of the ascending aorta is not seen (1). In this view, though flow is not completely parallel with the Doppler beam, it is still possible to interrogate ascending flow. Aortic flow patterns are very important to differentiate abnormal pathology from imaging artifacts.
 FIGURE 17.2. Midesophageal (ME) ascending short-axis view (SAX). RPA, right pulmonary artery; SVC, superior vena cava; PA, pulmonary artery; AO, ascending aorta. |
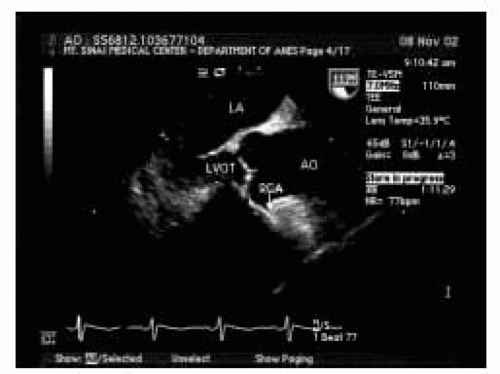 FIGURE 17.3. ME ascending aorta long-axis view (LAX). LA, left atrium; LVOT, left ventricular outflow tract; RCA, right coronary artery; AO, aorta. |
After imaging the ascending aorta, it is usually easiest to image the descending aorta. The descending aorta is located by starting in the ME four-chamber view and rotating the shaft of the echo probe 180 degrees. Once the descending aorta is visualized, adjust the depth setting to center the aorta in the image. At this point, it is also important to set the probe at the highest imaging frequency to obtain the best resolution. Insert the probe to the level of the diaphragm where the image of the descending aorta disappears. Then slowly withdraw the probe while keeping the image centered with slight rotational adjustments. The entire descending aorta can be interrogated in this fashion. These short-axis views are useful to define the wall thickness and tissue characteristics, aortic dimensions, and aortic flow patterns (Fig. 17.4). It is also possible to detect left-sided pleural effusions and atelectasis in these views. The aorta should imaged in the same fashion in the 90-degree imaging plane to obtain long-axis views of the descending aorta (Fig. 17.5). The long-axis views are most useful for defining the presence of branch vessels and the spatial relationship of the short-axis findings. Flow patterns are also very useful in these images.
The aortic arch is most easily located at the end of the interrogation of the descending aorta as the probe is being withdrawn. At the arch in the 0-degree imaging plane, the short-axis view of the aorta will transition to a long-axis view—the UE aortic arch LAX view (Fig. 17.6). To optimize visualization of the arch, it is often necessary to rotate the shaft of the probe in a clockwise direction. It may also be helpful to manipulate the degree of flexion in
the tip. The LAX view is useful for defining the aortic dimensions, wall contour, and branch vessels. The innominate vein is often seen in this view. After the visualization in the long axis is complete, to obtain the short-axis views of the arch, it is necessary to rotate the imaging plane to 90 degrees (Fig. 17.7). Starting at distal arch, multiple short-axis views of the arch and branch vessels can be obtained by clockwise rotation of the shaft of the probe. At the proximal portion of the arch the main pulmonary artery and left pulmonary may be visualized. In both the LAX and SAX views it is important to consider the wall thickness and tissue characteristics, aortic dimensions, and blood flow patterns. Even using all of these views, it is still not possible to image the entire thoracic aorta. In addition to the blind spot in the distal ascending aorta created by the airway, there are limited LAX and SAX scan planes of the ascending aorta. Additionally, the aorta is subject to many types of artifacts. To overcome these problems during surgical procedures with a sternotomy, it is possible to image the entire ascending aorta by epiaortic echocardiography. Epiaortic scanning can be performed with either sector or linear probes. The sector probes have a smaller footprint and are easier to move within the chest, but have poorer lateral resolution. On the other hand, linear probes have a larger footprint but offer better lateral resolution. In rare circumstances, it may be desirable to place a probe under the arch and this can be accomplished with a transesophageal probe. All of these probes must be sterilely wrapped. Other than the theoretical possibility of introducing contamination and the necessary 5 to 10 minutes for the examination, there are no known complications of epiaortic scanning. However, there are several important imaging considerations.
the tip. The LAX view is useful for defining the aortic dimensions, wall contour, and branch vessels. The innominate vein is often seen in this view. After the visualization in the long axis is complete, to obtain the short-axis views of the arch, it is necessary to rotate the imaging plane to 90 degrees (Fig. 17.7). Starting at distal arch, multiple short-axis views of the arch and branch vessels can be obtained by clockwise rotation of the shaft of the probe. At the proximal portion of the arch the main pulmonary artery and left pulmonary may be visualized. In both the LAX and SAX views it is important to consider the wall thickness and tissue characteristics, aortic dimensions, and blood flow patterns. Even using all of these views, it is still not possible to image the entire thoracic aorta. In addition to the blind spot in the distal ascending aorta created by the airway, there are limited LAX and SAX scan planes of the ascending aorta. Additionally, the aorta is subject to many types of artifacts. To overcome these problems during surgical procedures with a sternotomy, it is possible to image the entire ascending aorta by epiaortic echocardiography. Epiaortic scanning can be performed with either sector or linear probes. The sector probes have a smaller footprint and are easier to move within the chest, but have poorer lateral resolution. On the other hand, linear probes have a larger footprint but offer better lateral resolution. In rare circumstances, it may be desirable to place a probe under the arch and this can be accomplished with a transesophageal probe. All of these probes must be sterilely wrapped. Other than the theoretical possibility of introducing contamination and the necessary 5 to 10 minutes for the examination, there are no known complications of epiaortic scanning. However, there are several important imaging considerations.
First, the depth and zoom settings should be optimized to maximize the aortic size. Second, it is necessary to create
a standoff from the aorta to the probe to visualize the anterior wall of the aorta. This can be accomplished with gel or water. The aorta is best interrogated in both the long- and short-axis imaging planes (Figs. 17.8 and 17.9).
a standoff from the aorta to the probe to visualize the anterior wall of the aorta. This can be accomplished with gel or water. The aorta is best interrogated in both the long- and short-axis imaging planes (Figs. 17.8 and 17.9).
 FIGURE 17.8. Epiaortic SAX view. ANT, anterior; AO, aorta. Note the hazy density on the left side of the image. This is a typical artifact seen in the aorta. |
STRUCTURES (NORMAL ANATOMY)
The aorta is the largest artery of the body. It contains a considerable amount of elastic tissue and smooth muscle in its wall to prevent disruption. The aortic wall is composed of three layers: intima, media, and adventitia. The intimal surface is a thinly lined layer with a confluent monolayer of flattened endothelial cells resting on a thin basal lamina. The endothelium regulates vasomotor tone, plays an intimate role in the coagulation cascade, and mediates inflammatory and immunologic responses. The media consists of smooth muscle cells enmeshed in a matrix of connective tissue components (collagen, elastin, and occasional fibroblasts) arranged in a laminated and intertwined fashion in a spiral pattern (2). This layer accounts for up to 80% of the aortic wall thickness and provides mechanical and structural support to the aorta along with vascular tone (3).
The adventitia is a looser layer of tissue containing collagen, lymphatics, and the vasa vasorum. It is responsible for providing nutrients to the aortic wall.
The aorta begins at the aortic valve and is divided into four parts:
1. Ascending aorta
2. Transverse aortic arch
3. Descending thoracic aorta
4. Abdominal aorta
The ascending aorta arises from the left ventricle posterior to the right ventricular infundibulum and the pulmonary valve. It courses superiorly and slightly rightward. The proximal portion of the aorta is comprised of the sinuses of Valsalva. These three sinuses bulge outward to allow full excursion of the aortic leaflets during ventricular systole and to accommodate the retrograde flow during diastole. The middle third of the ascending aorta is to the right of the main pulmonary artery, anterior to the right pulmonary artery, and anteromedial to the superior vena cava. The distal third of the ascending aorta courses superiorly, and runs posteriorly and leftward at the level of the aortic arch. This part of the aorta lies anterior to the trachea and the right mainstem bronchus, superior to the pulmonary artery, and posterior to the innominate vein.
The aortic arch gives rise to three branches from its superior aspect:
1. The innominate (brachiocephalic) artery
2. The left common carotid artery
3. The left subclavian artery.
All three branches course posterosuperiorly. The innominate and the left common carotid arteries are in close proximity to the anterior aspect of the trachea, while the left subclavian artery lies to the left of the trachea. The descending thoracic aorta begins distal to the left subclavian artery at the level of the ligamentum arteriosum. This area is referred to as the aortic isthmus. At the distal portion of the aortic arch and the beginning of the descending thoracic aorta, the aorta courses caudally and lies in close proximity to the esophagus in the left thoracic cavity. As the aorta descends in the thoracic cavity, it curves to course posteriorly to the esophagus. Finally, at the level of the distal portion of the descending thoracic aorta, the aorta will lie directly posterior to the esophagus.
PATHOLOGY
This section will focus on three main diseases of the aorta: atherosclerosis, aortic aneurysm, and aortic dissection. One of the important considerations in the evaluation of aortic pathology is the differentiation of pathology from imaging artifact. So this section will also illustrate some of the common imaging artifacts encountered in the interrogation of the aorta.
Atherosclerosis
Neurologic injury after procedures with cardiopulmonary bypass (CPB) continues to be a major problem and occurs in 1%-10% of the patients (4). Because there is very little one can do to treat this injury, prevention of injury is the only real option. It is unclear which factor or factors are primarily responsible for this neurological injury after CPB; however, cerebral embolization and cerebral hypoperfusion continue to be the major focus of study. The watershed zones of infarction in the brain are areas thought to be at high risk for ischemic injury from decreases in perfusion pressure due to their position at the border of two distinct territories of perfusion. Because these areas rely on small arteriolar connections, they are subject to malperfusion based on decreased perfusion pressure, but they are also susceptible to embolic events based on their small caliber and their location as branches of major arteries. Retinal microvascular lesions, as well as pathologic subcapillary arteriolar dilatations in the brain have been demonstrated after CPB.
Prior investigations that focused on cerebral hypoperfusion, nonpulsatility, and air embolization as the major etiologic factors for stroke have been supplanted by studies of atheroemboli, which are now believed to be responsible for a majority of cerebral embolic events during open or closed cardiac procedures (5,6,7,8,9). Valvular surgery and open cardiac procedures were once thought to be higher risk procedures for micro- and macroembolization because of the entrainment of air into cardiac chambers and the potential for embolization of calcific and thrombotic debris (10). Atherosclerosis of the ascending thoracic aorta and aortic arch is now recognized as one, if not the major predictor of postoperative stroke after cardiac surgery (11). Additional evidence for the role of aortic atherosclerosis was provided by an autopsy study by Blauth et al. (n = 221) (12). The study found that atheroemboli were more common after coronary revascularization than after valvular procedures, p = 0.008. Peripheral vascular disease and ascending aortic atherosclerosis were significant independent risk factors for atheroemboli. A direct correlation between age and ascending aortic atherosclerosis was found, as well as a high correlation between ascending aortic atherosclerosis and atheroembolic events. Of the 98 patients who did not have atherosclerosis of the ascending aorta only 2 had autopsy evidence of embolic phenomena. Forty-six of the 123 patients with atherosclerosis of the ascending aorta had autopsy evidence of embolic events.
When aortic instrumentation is planned, the presence of ascending, transverse, and descending aortic atheromatous disease is a harbinger of potential cerebral embolization. Using echocardiographic techniques, aortic atherosclerosis can and should be diagnosed prior to anticipated instrumentation. Ascending aortic plaque is often soft, friable, and not able to be palpated by the cardiac surgeon. A number of investigations have shown that palpation underestimates the incidence of aortic plaque when compared with diagnosis via echocardiographic techniques (13,14). Its presence may only be recognized after it is seen oozing from an aortotomy site. After aortotomy, the embolization of atherosclerotic debris may have already occurred with resultant adverse sequelae. Thus, the preoperative identification of aortic plaque, made possible by improved echocardiographic technologies, has become an area of intense interest and research.
Risk Factors
In order to accurately assess clinical risk for the development of stroke (stroke having an incidence of only 1%), sufficient numbers of patients must be studied in order to attain statistical power. For this reason, many of the studies that have identified risk factors have been necropsy studies, retrospective chart review, or prospective multicenter studies. Additionally, different criteria for diagnosis and assessment make comparison of studies quite difficult.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree