Assessment of the Mitral Valve in Ischemic Heart Disease1
Alexander N. Chapochnikov
Solomon Aronson
David Jayakar
Mitral regurgitation (MR) that accompanies acute myocardial ischemia or infarction is associated with an unfavorable prognosis for both medical and surgical treatment. It has been reported that up to 41% of coronary artery bypass-grafting (CABG) candidates may also have chronic ischemic mitral insufficiency (1). When the severity of ischemic mitral regurgitation (IMR) necessitates CABG and mitral valve repair or replacement, the combined procedure may lead to significantly increased in-hospital mortality compared with that for CABG alone. This risk is further enhanced in patients over 80 years of age (2). It has previously been demonstrated that CABG alone can lead to reduction of IMR severity (3). On the other hand, significant residual MR after revascularization is associated with increased immediate postoperative and long-term morbidity and mortality (4,5).
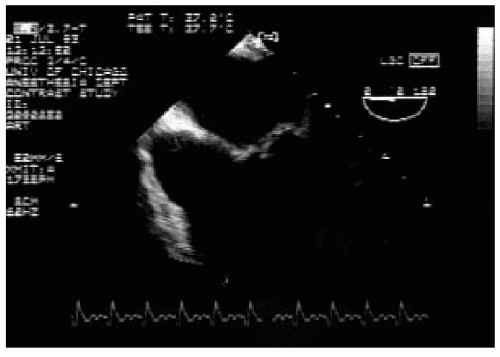
The intraoperative severity assessment of residual MR is further complicated by hemodynamic changes related to the effect of general anesthesia and is often underestimated (6). Intraoperative transesophageal echocardiography (TEE) in experienced hands enables precise assessment of the mechanism and may improve intraoperative decision making and patient outcome. This chapter discusses the anatomic and physiologic principles of IMR and reviews the use of TEE in the diagnosis of this condition (7,8).
SCIENTIFIC PRINCIPLES
Prevalence and Outcome
Ischemic MR is present in 7% to 31% of patients undergoing coronary angiography (4,9). In a series of 140 patients with mitral insufficiency, 26% had IMR (10), with other reported causes of MR in that series being 41% myxomatous degeneration, 17% rheumatic valvular disease, 12% endocarditis, and 2% congenital pathology (Fig. 25.1). The same group reported that among 755 patients who had undergone a primary operation for MR (11), the total operative mortality rate was 4%, whereas the mortality rate in the group of patients with combined repair and CABG was 6.6%. Analysis of 150 consecutive patients with IMR undergoing either repair (63%) or replacement has been reported to result in different long-term outcome based on the underlying pathophysiology, rather than the type of procedure (12). It was found that the functional subset of IMR (annular dilatation or restrictive leaflet motion) had the worse long-term (5-year) survival rate (43%) compared with the structural IMR (ruptured chordae or papillary muscle) repair (76%) or structural/replacement groups (89%). The predictor of worse long-term survival (repair of functional IMR) indicates that pathophysiologic mechanisms may be the major determinants of survival rather than the type of surgical
intervention (12). Retrospective analysis of 1,292 patients at the Cleveland Clinic over a 5-year period revealed an overall incidence of IMR of 6.5%, of which 40% had valve prolapse and 60% had restrictive leaflet motion due to regional or global left ventricular (LV) dilatation (13). Forty-two percent of the patients had rheumatic valvular disease; 44% had degenerative valvular disease; and 6% had endocarditis or congenital valve malformation. A mean follow-up (3 ± 1.6 years) interval after repair revealed a superior survival in the patients with valve leaflet prolapse (96%) versus 48% for those with restricted valvular motion (13,14).
intervention (12). Retrospective analysis of 1,292 patients at the Cleveland Clinic over a 5-year period revealed an overall incidence of IMR of 6.5%, of which 40% had valve prolapse and 60% had restrictive leaflet motion due to regional or global left ventricular (LV) dilatation (13). Forty-two percent of the patients had rheumatic valvular disease; 44% had degenerative valvular disease; and 6% had endocarditis or congenital valve malformation. A mean follow-up (3 ± 1.6 years) interval after repair revealed a superior survival in the patients with valve leaflet prolapse (96%) versus 48% for those with restricted valvular motion (13,14).
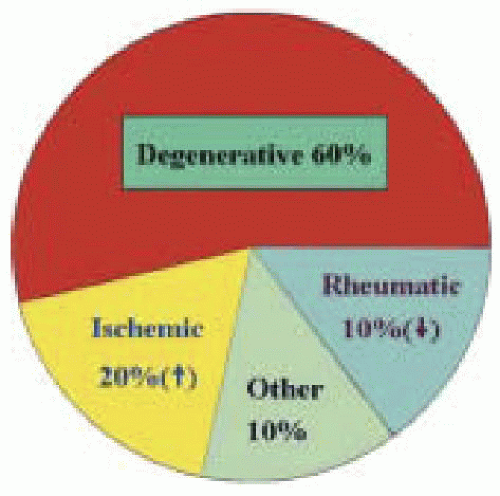 FIGURE 25.1. Prevalence of mitral valve disease. Ischemic etiology is increasing while rheumatic etiology is decreasing in the U.S. population. |
In a reported series by Seipelt and colleagues, 262 patients underwent mitral valve operations (replacement, 198; repair, 64) in combination with coronary revascularization. MR was determined to be secondary to coronary artery disease (CAD) in 31% of patients (19.5% in-hospital mortality), whereas 53% of cases had a rheumatic etiology and 16% were attributed to degenerative changes, with a combined in-hospital mortality rate of 6.7% (15). Mitral valve repair was performed in 37% of the patients with IMR versus 19% with rheumatic or degenerative disease. The survival rates (valve-related, event-free survival) in the first, fifth, and tenth years were 94%, 66%, and 53% in the IMR group, compared with 95%, 76%, and 41% in the rheumatic or degenerative groups.
The reported in-hospital mortality rate for isolated CABG is 3%, whereas isolated mitral valve procedures carry a reported in-hospital mortality rate of 3% to 7% (16,17).
Combined mitral valve surgery and CABG is associated with a reported in-hospital mortality rate of 7% to 20% (2,16,17,18), with advanced age of the patients (≥ 80 years) being described as an independent risk factor leading to increased in-hospital mortality (19.6% vs. 12.2% in younger patients) as well as congestive heart failure (CHF), acute onset of ischemia requiring intensive care, LV end-diastolic pressure greater than 15 Torr, and New York Heart Association class IV (2,8).
Investigators have reported that patients with grade 2 or less ischemic MR (group I) who underwent CABG alone had reduced angina pectoris and improved functional status equal to CABG patients without MR (group II) (19). In that series, IMR patients were older and had lower preoperative LV ejection fraction (42% vs. 58%). The 30-day mortality rates were similar (4.5% in both groups), as were the survival rates at 1 year (91% vs. 93%) and 3 years (84% vs. 88%). The severity grade of MR increased in 2% of the patients and was unchanged in 36% (19).
At the Society of Cardiovascular Anesthesiologists (SCA) Annual Meeting in April 2002, it was reported that isolated off-pump coronary artery bypass (OPCAB) may offer improvement and resolution of mild IMR (20). A total of 144 patients undergoing OPCAB were evaluated retrospectively. Sixteen of 144 required conversion to cardiopulmonary bypass (CPB), and 5 patients among them had moderate IMR. Of the remaining 128 patients who tolerated the OPCAB procedure, 109 were examined by preoperative, intraoperative, and postoperative echocardiography. Fourteen patients were excluded from the study because nonischemic mitral valve pathology was found. Forty-eight of 109 patients (44%) had varied degrees of IMR, 11 patients (10%) had moderate IMR, 37 patients (34%) had mild or trace IMR, and none had severe IMR. Three of five patients with moderate IMR who required conversion to CPB died postoperatively due to low output syndrome and respiratory failure. Postoperative transthoracic echocardiography (TTE) or TEE revealed no improvement in IMR degree in four of these five patients. The patients with moderate IMR who tolerated OPCAB had postoperative TTE at 1 to 35 weeks, which showed improvement in MR severity in 9 of 11. All had functional improvement. No deaths were reported in the moderate IMR OPCAB group. The researchers concluded that OPCAB patients with moderate IMR who required conversion to on-pump CABG represented a highrisk group, whereas patients with moderate or less IMR who tolerated OPCAB appeared to benefit from revascularization alone. This may be important in the subgroup of patients with IMR and perioperative risk, which strongly preclude aortic occlusion and initiation of CPB (20).
Anatomy
The mitral valve (MV) is a complex structure consisting of two leaflets (posterior and anterior), the chordal structures (primary, secondary, and tertiary), the papillary muscles [anterolateral (AL) and posteromedial (PM)], and the ventricular wall extending between the base of the PM papillary muscle insertion and the annulus of the MV. While the LV remains in systole, the leaflets come together, forming the coaptation point, which—along with AL and PM commissures—create a “line of coaptation.”
The posterior leaflet consists of three scallops: medial, middle, and lateral. The corresponding portions of the anterior leaflet are medial, middle, and lateral thirds. The chordae, which consist of connective tissue (mainly collagen bundles), extend from the head of each papillary muscle to the nearest half of each leaflet.
The primary chordae attach to the tip of the leaflet, the secondary chordae attach to the mid-portion of the leaflet, and the tertiary chordae attach to the base of the leaflet or mitral annulus. These tertiary chordae (also referred to as “stay” chordae) form a posterior chordal structure that has a stabilizing function. Interruption of the tertiary chordae due to ischemic/necrotic rupture in the corresponding myocardial zone (including papillary muscle) will have a significant impact on the mainte
nance of normal LV geometry and ventricular function. The number of papillary muscle heads varies from one to five.
nance of normal LV geometry and ventricular function. The number of papillary muscle heads varies from one to five.
The blood supply to the PM papillary muscle was provided by one vessel (either right coronary artery or obtuse marginal artery) in 63% of the patients and two vessels in 37% (21). The AL papillary muscle had a double-vessel blood supply (obtuse marginal and diagonal) in 71% and single-vessel supply in 29%. In a study that included 20 patients monitored by TEE during coronary surgery, selective coronary graft injections of sonicated albumin microbubbles were performed to assess graft patency and papillary muscle perfusion. It was demonstrated that in the subgroup of 10 patients with an old inferior myocardial infarction (MI), MR was present only among those 6 with a single rather than a double blood supply.
Presently used nomenclature systems include both Carpentier classification, where the posterior MV leaflet is divided into P1, P2, and P3 (lateral, middle, and medial) scallops, and the Duran classification (22). The latter uses a P1, PM, P2 for the same scallops, respectively, where the middle scallop is subdivided into the PM1 and PM2 with regard to the chordae that come from the corresponding AL papillary muscle (PM I) or the PM papillary muscle (PM2). The anterior leaflet (comprising 60%-70% of the mitral valve area and 30% of the annular circumference) is also divided differently. The Carpentier classification provides Al, A2, and A3 nomenclature (lateral, middle, medial) segments, respectively, versus a division into Al and A2 portions (by Duran classification) with the corresponding papillary muscles attached via chordae. The specific approach to the anterior leaflet division may reflect different mitral regurgitant pathophysiology and a different approach from the surgical standpoint. The American Society of Echocardiography and the SCA incorporate the anatomic classification represented by the Carpentier scheme. Mitral valve regurgitation may be caused by malfunctioning of any component of the mitral valve apparatus. Chronic regurgitation leads to dilatation of the left atrium (LA) and the annulus, which loses its physiologic elliptoid shape during systole. The resulting circular annulus portends poor leaflet coaptation and MR. Increased LA dimension suggests an advanced degree of MR, although smaller dimensions do not exclude insufficiency.
Mechanism
Mitral regurgitation as a primary complication of ischemic heart disease was initially described by Burch and De Pasquale in 1963, when they recognized papillary muscle dysfunction as a primary consequence of ischemia (23).
There are many ways to classify IMR, for example, acute versus chronic, or by underlying pathology, such as CAD with annular dilatation, CAD with ischemic or infarcted papillary muscles, or CAD with restrictive chordae or leaflet pathology with or without annular dilatation (24). The classification of mitral valve insufficiency proposed by Alain Carpentier included a description as follows: Type I: pure dilatation of the annulus, leaflet motion normal, leaflet perforation may be present; Type II: leaflet prolapse due to chordal rupture or elongation or papillary muscle rupture or elongation; and Type III: restricted leaflet motion due to posterior papillary muscle dysfunction in conjunction with LV dysfunction (25).
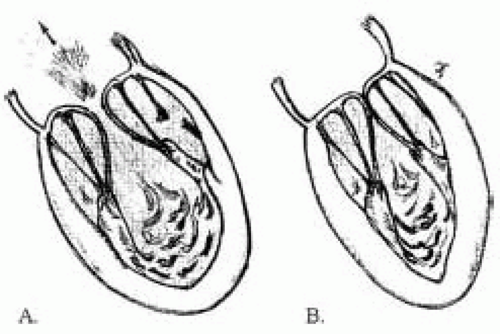
Acute transient MR during ischemic episode occurs primarily due to leaflet restriction as a result of ventricular dyskinesia, rather than of annular dilatation (26). Segmental asynergy of the LV wall was found in up to 96% of
the patients with acute severe MR. The inferior wall was impaired in 86% (Fig. 25.2) (27).
the patients with acute severe MR. The inferior wall was impaired in 86% (Fig. 25.2) (27).
It is important to distinguish IMR that is described in the context of other organic causes of MR from the IMR described among patients with CAD and no primary organic mitral valve disease. The latter type may be as high as 57%, with prevalence up to 90% represented by incomplete mitral leaflet closure in patients with acute MI and acute IMR (14).
The current understanding about the mechanism of IMR after acute MI involves consideration of unbalanced ventricular forces, changes in papillary muscle geometry, asymmetric widening of the annulus, expansion of the border zone myocardium, and early systolic leaflet loitering.
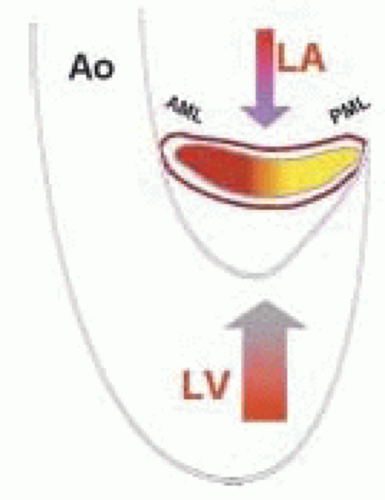
The normal saddle shape of the valve promotes caudal opening, with less force required to open the valve than to close it (Fig. 25.3) (28). The diastolic LA pressure to LV pressure gradient determines the rate of mitral valve leaflet opening. The rate of change of the early systolic LV to LA pressure gradient affects (in part) the degree of mitral leaflet closure. If this gradient is low (as seen with LV dysfunction), then MR will occur. This principle was examined by Dent et al. (28) when global LV function was changed by altering the left main coronary artery flow while maintaining constant LA pressure. Left ventricular end-systolic dimension and peak positive LV rate of pressure development (dP/dt) correlated well with the degrees of mitral leaflet opening and closure. They concluded that LV systolic function determines the extent (both opening and closure) of mitral leaflet excursion (28).
Papillary muscle dynamics also play a significant role in proper mitral leaflet closure in normal hearts. In a diseased heart, even a preexisting single blood supply to the PM papillary muscle can uncommonly lead to its necrosis. On the other hand, the consequent LV systolic dysfunction of ischemia may significantly contribute to the geometry changes of the mitral valve apparatus. The tethering distance (Fig. 25.4) between an ischemic PM papillary muscle tip and the anterior annulus has been measured experimentally. Initial inferior ischemia alone produced papillary muscle tip retraction with restricted closure and mild to moderate MR (regurgitant fraction, 25%), whereas the addition of papillary muscle ischemia consistently decreased MR and tethering distance. It was concluded that papillary muscle contractile dysfunction paradoxically decreased MR because the inferobasal ischemia reduced leaflet tethering and improved coaptation (29).
The otherwise normal constant distance between the tip of the papillary muscle and the annulus may become variable in ischemic heart disease, whereas the papillary muscle tip to the leaflet edge distance (determined by the length of the primary chorda) remains constant despite the presence of ischemic changes. If the primary chorda is intact, tethering of the leaflet edges occur, which leads to restrictive leaflet closure and MR (Fig. 25.5). The annulus area normally decreases by 25% during systole and preserves its physiologic elliptoid shape. Most changes in annulus shape are posterior.
Komeda and associates used an acute ischemic dog model along with radiopaque markers and simultaneous
biplane videofluoroscopy to evaluate annular dimensions during regional (posterolateral wall) LV ischemia (30). They demonstrated that end-systolic mitral annulus area increased (4.9 vs. 5.9 cm2) and IMR occurred in an asymmetric manner, with the most anterior annular segment lengths not changed. Markedly increased radial dislocation of PM papillary muscle at end-systole, dilatation (in the septal-lateral direction) of the mitral annulus, and AL apical motion (tethering) of the posterior mitral leaflet all led to MR. Based on these data, it might seem that an initial procedure of choice would be a ring annuloplasty provided that TEE assessment revealed no leaflet prolapse. The ring annuloplasty, although causing augmentation of leaflet coaptation, may in some circumstances not completely eliminate MR due to the fact that it does not normalize the radial displacement of the posterior mitral leaflet and the PM papillary tips in the radial direction. Thus, the tethering effect may not be eliminated, and an additional procedure, such as chordal extension (or replacement with expanded synthetic chordae), posterior papillary muscle elongation, or even excision of the posterior LV wall between the PM bases may be necessary.
biplane videofluoroscopy to evaluate annular dimensions during regional (posterolateral wall) LV ischemia (30). They demonstrated that end-systolic mitral annulus area increased (4.9 vs. 5.9 cm2) and IMR occurred in an asymmetric manner, with the most anterior annular segment lengths not changed. Markedly increased radial dislocation of PM papillary muscle at end-systole, dilatation (in the septal-lateral direction) of the mitral annulus, and AL apical motion (tethering) of the posterior mitral leaflet all led to MR. Based on these data, it might seem that an initial procedure of choice would be a ring annuloplasty provided that TEE assessment revealed no leaflet prolapse. The ring annuloplasty, although causing augmentation of leaflet coaptation, may in some circumstances not completely eliminate MR due to the fact that it does not normalize the radial displacement of the posterior mitral leaflet and the PM papillary tips in the radial direction. Thus, the tethering effect may not be eliminated, and an additional procedure, such as chordal extension (or replacement with expanded synthetic chordae), posterior papillary muscle elongation, or even excision of the posterior LV wall between the PM bases may be necessary.
The Surgical Anterior Ventricular Endocardial Restoration (SAVER) trial has demonstrated that surgical anterior ventricular endocardial restoration can be used for remodeling the dysfunctional and dilated anterior portion of the LV after anterior MI (31). In this important series of 439 patients, concomitant CABG was performed in 89% and mitral repair in 22%. Mitral valve replacement was necessary in 4% of the patients.
Gorman and associates in an acute infarction sheep model demonstrated that circumflex coronary artery occlusion (which subtended 32% of the posterior LV) produced acute 2 to 3+ MR. Sonomicrometry transducers implanted around the mitral annulus and the tips and bases of papillary muscles revealed that the mitral annulus was dilated asymmetrically orthogonal to the line of leaflet coaptation. Although annular area increase was rather small (9.2%), when combined with tethering of the leaflet scallops, it was sufficient to produce moderate to severe MR (32).
Radiopaque markers sutured around the mitral annulus, to the central free mitral leaflet edges, and to both papillary muscle tips and bases again helped reveal changes in mitral annular dimensions and leaflet closing dynamics in pre- and postinduction of acute ischemia (33). During control (preischemic phase), leaflet coaptation occurred 23 msec after end-diastole, whereas during ischemia (with LV dysfunction) coaptation was delayed to 115 msec after end-diastole with MR occurring. Mitral annulus area was also 14% larger during ischemic conditions compared with control measurements. In that experimental model, the posterior papillary muscle tip was displaced laterally and posteriorly, but no apical displacement was noted. “Loitering” of the leaflets associated with posterior mitral annulus enlargement and circularization led to incomplete mitral leaflet coaptation during early systole, not at end-systole (33). This early systolic mitral annular dilatation and shape change, as well as altered posterior papillary muscle motion, should be considered as one of the mechanisms by which incomplete mitral leaflet coaptation occurs during acute IMR.
Carpentier and associates showed that mitral valve incompetence could not be produced by infiltrating the papillary muscle with formaldehyde. In order to get MR, it was also necessary to infiltrate the myocardial wall that supports the papillary muscle. These experimental findings were revealed when Carpentier commented on a similar conclusion made by Llaneras et al. (34).
The role of an LV shape in the development of functional MR in heart failure was demonstrated by Sabbah and associates (35). Global LV shape changes were induced in dogs by multiple sequential intracoronary microembolizations. Sixty-one percent of animals developed 1+ to 3+ MR during heart failure. End-systolic sphericity index increased 72%. Animals without MR were found with only a 30% increase of the same index. These data support the concept of spherical LV shape as a determinant of functional MR (35,36,37). The same group of researchers (35) commented on the mechanism of functional MR with the emphasis on the LV chamber enlargement and LV reshaping along with papillary muscle dysfunction, regional LV wall motion abnormalities, and dilatation of the mitral valve annulus (38).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree