Spatial Area Mapping
Color flow Doppler is one of the most commonly used semiquantitative methods to estimate the severity of mitral regurgitation (
21). Color flow Doppler is based on pulsed wave ultrasound techniques with different signal processing and display formats. Instead of measuring velocities at a single location as with pulsed wave Doppler, color flow Doppler has a number of gates positioned at different depths along many scan lines. Velocity
is encoded into different colors based on the direction of flow to or away from the transducer (
22). The extent of the velocity map displayed by color flow Doppler is reflective of the velocity of regurgitant flow rather than absolute regurgitant volume (
18). In general, color Doppler can quickly differentiate mild degrees from severe grades of mitral regurgitation (
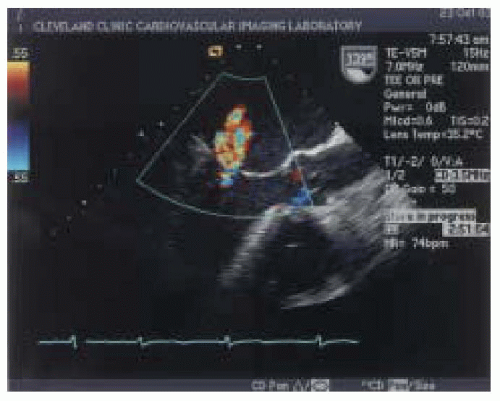
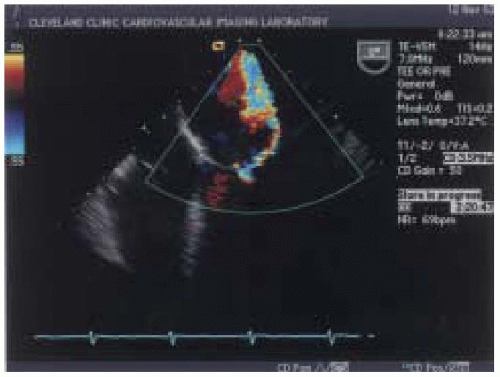
Figs. 14.5 and
14.6).
Color flow Doppler estimates of mitral insufficiency correlate well with the semiquantitative angiographic grades of insufficiency (
21). Castello and colleagues compared the correlation between color flow Doppler regurgitant jet area measurements to angiography. A maximal jet area < 3 cm
2 predicted mild mitral regurgitation with a sensitivity of 96%, a specificity of 100%, and a predictive accuracy of 98%; whereas a maximal regurgitant area of > 6 cm
2 predicted severe regurgitation with a sensitivity of 91%, specificity of 100%, and predictive accuracy of 98% (
23).
Spain and colleagues also reported a good correlation between maximal color flow Doppler jet area and angiographic grades of mitral insufficiency. However, they reported limited correlation between quantitative measurements of mitral regurgitant severity, such as regurgitant volume and regurgitant fraction, with maximal jet area measurements (
24).
Rivera and colleagues compared a
visual assessment method, the color flow Doppler jet area method, and regurgitant fraction measurements for grading the severity of mitral regurgitation. The visual assessment method encompassed integrating information about actual jet dimensions and jet eccentricity as well as chamber geometry to provide an educated assessment of the degree of regurgitation present. They reported that the visual grading method had a better correlation with quantitative measures of regurgitation than jet area measurements (
25).
Several technical factors influence the appearance of the color flow signal within the left atrium. Among these are instrumentation settings, such as frame rate, gain settings, and transducer frequency (
22). Alterations in colorscale settings impact the effect of entrainment of left atrial blood on the regurgitant jet area. Setting the color scale to the highest possible level will limit the effect of entrainment (
26). Maintaining constant technical factors reduces instrumentation errors.
Alterations in intraoperative hemodynamics also influence the jet of mitral regurgitation as detected by color flow Doppler (
27,
28). Grewal and colleagues (
27) reported that slightly more than half of patients with mitral insufficiency improved at least one grade with the induction of general anesthesia. Decreased intravascular volume coupled with a reduction in afterload was thought to contribute to better leaflet coaptation and reduced valvular insufficiency (
27).
Compliance and size of the receiving chamber confound the relationship between the size of the regurgitant jet and regurgitant volume (
24). Patients with acute, severe mitral regurgitation may display a relatively small jet area secondary to high left atrial pressures due to limited compliance of the left atrium (
18).
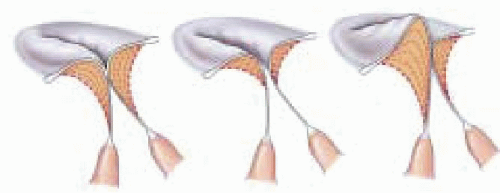
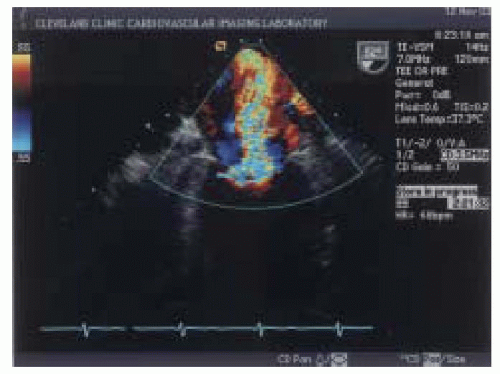
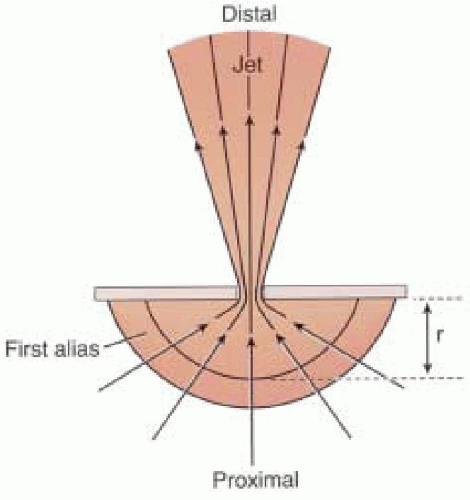
Eccentric regurgitant jets when imaged by color-flow mapping commonly occupy less overall area compared to jets of similar flow rates directed centrally within the left atrium (
29). An eccentric jet has a different observed morphology as compared to free jets secondary to limited expansion due to impingement of the jet along the atrial wall. Consideration of jet morphology in the color flow
Doppler assessment is important to avoid underestimating the degree of regurgitation (
Fig. 14.7) (
29).
Pulmonary Venous Waveform Patterns
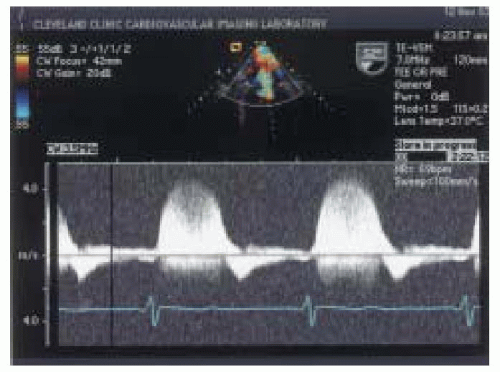
Normal pulmonary venous waveform patterns consist of a biphasic forward systolic waveform occurring during ventricular systole, a forward diastolic velocity waveform that occurs after mitral valve opening, and a retrograde atrial flow reversal waveform that occurs in response to atrial contraction (
30). In general, the normal pattern of pulmonary venous flow displays the ratio of systolic waveform to diastolic waveform as greater than or equal to one. Current applications for pulmonary venous waveform patterns include differentiating constrictive pericarditis from constriction, estimation of left ventricular filling pressures, evaluation of left ventricular diastolic dysfunction, and grading the severity of mitral regurgitation (
31).
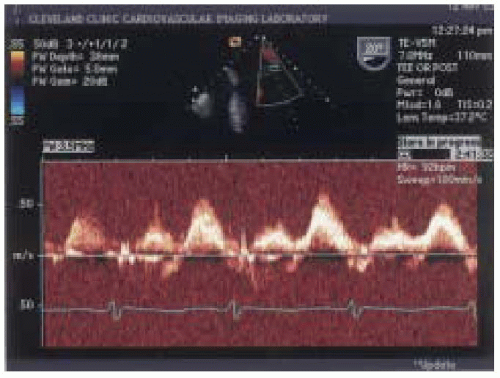
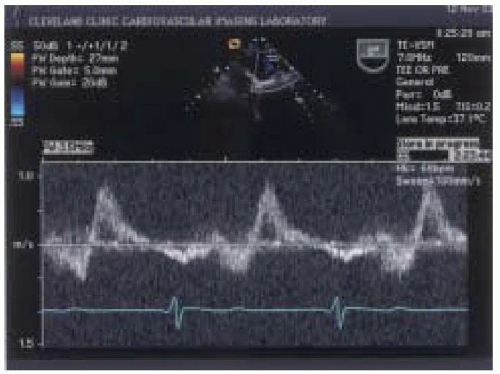
Significant degrees of mitral regurgitation increase left atrial pressure and alter forward flow through the pulmonary veins. Klein and colleagues (
32) investigated the relationship between the ratio of peak systolic and peak diastolic flow velocities in the pulmonary veins to varying degrees of mitral regurgitation as measured with TEE color-flow mapping. The ratio of peak systolic and diastolic pulmonary venous waveform velocities were categorized as having a normal pattern where the ratio of peak systolic/diastolic waveform was greater than or equal to one, a blunted pattern where the ratio of peak systolic to peak diastolic waveform was between 0 and < 1 (
Fig. 14.8), and reversed systolic waveform represented by a peak systolic velocity value of < 0 (
Fig. 14.9). The sensitivity and specificity for reversed systolic flow detecting 4-plus mitral regurgitation was 93% and 100%, respectively. Blunted systolic flow for detecting 3-plus mitral regurgitation had lower sensitivity and specificity of 61% and 97%, respectively (
32).
Pulmonary venous flow patterns may not be a reliable marker of valvular insufficiency for all grades of mitral regurgitation. Pu and colleagues evaluated the relationship between pulmonary venous flow patterns and quantitative indexes of mitral regurgitation in patients with variable degrees of left ventricular function. Quantitative Doppler
measurements included regurgitant orifice area, regurgitant stroke volume, and regurgitant fraction measurements. A normal pulmonary venous waveform pattern had a sensitivity, specificity, and predictive value for detecting a small regurgitant orifice area of less than 0.3 cm
2 of 60%, 96%, and 94%, respectively. The reversed pattern was a highly specific marker for detecting a large regurgitant orifice area of greater than 0.3 cm
2 with a sensitivity, specificity, and predictive value of 69%, 98%, and 97%, respectively. The blunted pattern was seen in all grades of mitral regurgitation and had low predictive value for grading the severity of mitral regurgitation, particularly in patients with left ventricular dysfunction (
33).
Hynes and colleagues reported similar results regarding usefulness of systolic flow reversal as an indicator of severe mitral regurgitation and a normal waveform pattern confirming the absence of significant mitral regurgitation. They reported that the blunted pulmonary venous waveform pattern was more likely associated with left ventricular abnormalities than mitral regurgitation (
34).
Pulmonary venous flow patterns are influenced by a number of factors that include changes in myocardial relaxation (
35), abnormal left ventricular compliance, systolic and diastolic dysfunction, changes in loading conditions, and left atrial compliance and function (
30,
31,
36,
37,
38,
39,
40,
41). Other cardiac states that alter pulmonary vein flows include arrhythmias, such as atrial fibrillation (
31,
42).
Klein and colleagues, in a separate investigation, highlighted the importance of sampling both pulmonary veins when grading mitral regurgitation by TEE. They assessed the variability between left and right pulmonary venous flow in the assessment of mitral regurgitation. Discordant pulmonary venous flow as measured by PW Doppler TEE occurred between the left and right upper pulmonary veins at a rate of 37% in those patients with 4-plus mitral insufficiency (
43).
Schwerzmann and colleagues reported that combined transmitral E wave velocity and reversed systolic pulmonary venous flow were accurate measures for the determination of moderately severe to severe mitral regurgitation as compared to regurgitant fraction. They reported that reversed systolic pulmonary venous flow with an increased E wave velocity of > 1m/s had the sensitivity of 78% and a specificity of 97% for detecting severe mitral regurgitation (
100).