Assessment of the Aorta
Marc Kanchuger
Ellise Delphin
The first successful reparative surgery of the thoracic aorta occurred between 1949 and 1953, without the benefit of sophisticated diagnostic equipment, or high-tech surgical and anesthetic techniques. Dubost was the first to successfully remove an abdominal aortic aneurysm and replace it with a homograft in 1951 (1). Gross (2), Swan (3), Lam (4), and DeBakey (5) accomplished the successful treatment of both coarctation and aneurysms of the descending aorta. Although the origin of extracorporeal circulation is difficult to trace, it is most often attributed to Gibbon (6). This innovation permitted the first successful resection and graft placement in the ascending aorta, followed by successful resection and graft placement of the aortic arch. Over the past four decades, major advances in diagnostic modalities, such as anesthetic management of myocardial, cerebral, and spinal cord preservation and surgical tactics, have dramatically improved survival and quality of life after aortic surgery. The refinement and expanded use of transesophageal echocardiography as a diagnostic modality for aortic disease has refined perioperative care. Preoperative diagnosis of aortic disorders has become less invasive due to the unique sensitivity and specificity of transesophageal echocardiography. Intraoperative diagnosis of atheromatous disease has enabled improved outcomes in cardiac surgery because it serves as a guide to operative technique.
CLASSIFICATION AND EPIDEMIOLOGY OF DISEASES OF THE AORTA
Diseases of the aorta are generally considered to fall into five major groups:
1. Aneurysms
2. Dissections
3. Traumatic lesions
4. Atherosclerotic disease, including penetrating althero-sclerotic ulcer and intramural hematoma
5. Other disease
Aneurysms
Description
Aneurysm is defined as a localized or diffuse aortic dilatation of more than 50% normal diameter (7). Dilatation is progressive and develops from weakening of the aortic-wall. Aneurysms may be congenital or acquired. The etiology of congenital aneurysms is often connective tissue disease, such as Marfan’s syndrome or Ehlers Danlos syndrome. Acquired aneurysms are more common and are often due to atherosclerotic disease in combination with degenerative changes of the media. Advanced age, smoking, and hypertension are often associated with this type of acquired aneurysm. In addition to atherosclerotic disease and cystic medial necrosis, acquired aneurysms may be caused by trauma, infection, inflammation, or iatrogenic causes. Sixty-five percent of aneurysms only involve the abdominal aorta, while 11% involve the thoracoabdominal aorta, and 6% affect the distal thoracic aorta and arch (8).
Epidemiology
The incidence and prevalence of thoracic aneurysms in the United States population are difficult to estimate. The best population-based study to date, by Bickerstaff et al. (9), reported the incidence of newly diagnosed thoracic aneurysms as 5.9 per 100,000 person-years. The lifetime probability of rupture is 75-80% with 5 year untreated survival rates in the range of 10 to 20%. In nondissecting abdominal aneurysms, size significantly influences median time to rupture with a 43% risk of rupture within one year for aneurysms greater than 6 cm, and an 80% risk with those greater than 8 cm (10). Juvonen described the natural history of thoracic and thoracoabdominal aneurysms in 114 patients. In this group maximal diameter of greater than 5.8 cm was an independent risk factor for rupture as were advanced age, pain, and chronic obstructive pulmonary disease (11).
Dissection
Description
Aortic dissection is one of the most serious forms of aortic disease often requiring emergency surgery and aggressive medical care. A tear in the intima allows blood to escape from the true lumen of the aorta, creating a separation of the intima from the media, resulting in a false lumen. By definition, the true lumen is surrounded by intima, and the false lumen lies between the dissected layers. The dissection may remain localized or may propagate longitudinally and distally.
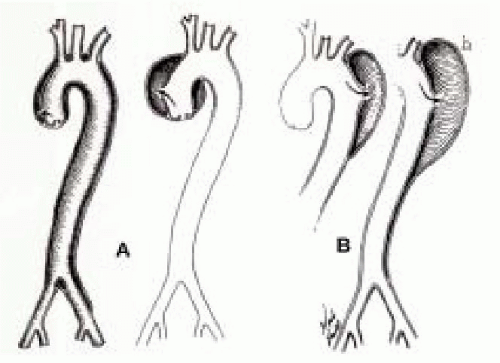
Two classification systems have been used to describe aortic dissections: the DeBakey system and the Stanford system (12,13). DeBakey classifies dissections into three types: Type I- intimal tear in the ascending aorta with extension of the dissection to the descending aorta, Type II-intimal tear in the ascending aorta with dissection confined to the ascending aorta, and Type III-tear beginning in the descending aorta (Fig. 31.1). The Stanford classification system is simpler and uses two groups: Type A dissections that involve the ascending aorta and Type B dissections involving only the descending aorta. The Stanford classification has become more popular because it is related to both therapeutic approach and risk. Type A dissections carry a mortality of 90% to 95% without surgical intervention and account for approximately 65% to 70% of all aortic dissections. Type B dissections carry a 40% mortality and medical management is the preferred type of therapy.
Epidemiology
The incidence of aortic dissection is approximately 5 per million population per year (14). Independent risk factors for aortic dissection include hypertension, advanced age, connective tissue disorders (Marfan’s syndrome), congenital diseases, such as coarctation of the aorta, trauma, and perhaps pregnancy. The disease predominantly affects men between 50 and 70 years of age.
Traumatic Aortic Disease
Description
Traumatic aortic injuries are the result of either blunt or penetrating trauma. Blunt (acceleration/deceleration) injuries occur from sheer forces that directly damage the arterial wall. The damage typically occurs at the transition point between the aortic arch, a fixed structure, and the more mobile descending aorta. These injuries are typical of motor vehicle accidents. Penetrating injuries occur secondary to stab and gunshot wounds.
Epidemiology
Of the 100,000 blunt chest trauma hospital admissions per year, approximately 3000 have aortic injuries requiring surgery (15). Patients with penetrating chest trauma have an incidence of aortic injury of approximately 4% (16). The survival of patients with either blunt or penetrating chest trauma is poor. Most of the patients die before reaching the emergency room. For those who reach the hospital, immediate diagnostic and surgical management are crucial.
Atherosclerotic Disease
Description
Atherosclerotic disease of the aorta is pathologically similar to this disease process in the other arteries of the body. Collections of macrophages laden with lipid develop into fibrous plaques that form focal lesions. Over time, they are covered by a layer of smooth muscle cells (17,18). Penetrating aortic ulcers (PAU) occur when an ulceration of an atherosclerotic plaque erodes into the media. This process may progress to the formation of aneurysm, dissection, aortic intramural hematoma (AIH), or aortic rupture. AIH has been defined as a localized separation of the layers of the aortic wall by partially or to-tally clotted blood, without an intimal tear. Due to their common clinical manifestations AIH, PAU, and aortic dissection have been called “acute aortic syndrome.” The rapid progression of both AIH and PAU to life-threatening disease warrants the same urgent approach to diagnosis as in aortic dissection. Patients with any of the three disorders may present with excruciating back pain and may progress to aortic rupture.
Lesions of the ascending aorta and arch have been identified as risk factors for stroke, peripheral embolization,
perioperative stroke as well as neuropsychological dysfunction after open heart surgery (19,20). Atheroemboli, thromboemboli, and plaque thickness: 4 mm correlate with embolic risk. Twelve percent of patients have recurrent strokes within a year, and 33% of patients have emboli. In addition, in patients undergoing cardiopulmonary bypass with identifiable aortic atheroma by transesophageal echocardiography, the risk of stoke is 12%, six times higher than the usual stroke rate.
perioperative stroke as well as neuropsychological dysfunction after open heart surgery (19,20). Atheroemboli, thromboemboli, and plaque thickness: 4 mm correlate with embolic risk. Twelve percent of patients have recurrent strokes within a year, and 33% of patients have emboli. In addition, in patients undergoing cardiopulmonary bypass with identifiable aortic atheroma by transesophageal echocardiography, the risk of stoke is 12%, six times higher than the usual stroke rate.
Epidemiology
Both aortic dissection and AIH seem to be widely distributed in all segments of the aorta. PAU tends to effect an older population with more cardiac risk factors and more widely spread disease. It occurs predominantly in the descending aorta. The natural history and management of AIH and PAU have not been defined.
OTHER DISEASE
Congenital Anomalies
Description
Coarctation of the aorta, anomalies of the arch and great vessels and patent ductus arteriosus are the most common adult congenital anomalies of the aorta. Coarctation of the aorta is the most common, and most likely to become problematic in adult life. It consists of a congenital narrowing of the aorta at the area of insertion of the ligamentum arteriosum. Diagnosis is made on physical examination. Hypertension is present in the upper extremities with weak or absent pulses in the lower extremities. Chronic increase in left ventricular afterload can result in hypertrophy or heart failure (24).
Epidemiology
Coarctation occurs in about 7% of all patients with congenital heart disease. It is twice as common in males as in females.
Connective Tissue Disease
Description
Marfan’s syndrome is an autosomal dominant connective tissue disorder that causes cystic medial necrosis of the aorta. Aortic dilatation and aneurysm result due to the weakened medial layer of elastin. The severe form of the disease is due to a mutation of a single allele of the fibrillin gene. Other connective tissue disorders, such as Ehlers-Danlos syndrome, often present in the same manner.
TUMOR
TRANSESOPHAGEAL ECHOCARDIOGRAPHY OF THE AORTA
Anatomy of the Aorta
Transesophageal echocardiographic evaluation, as well as anesthetic and surgical perioperative management depends not only upon the etiology of disease but also upon the segment of the aorta involved. The aorta is divided into four parts:
1. Ascending aorta
2. Transverse aortic arch
3. Descending thoracic aorta,
4. Thoracoabdominal aorta
The aortic root, the proximal third of the ascending aorta, begins at the aortic valve and houses the sinuses of Valsalva and the right and left coronary arteries. The root is particularly vulnerable in Marfan’s syndrome. It courses posterior, superior, and rightward to the right ventricular infundibulum and the pulmonary valve. The middle third of the ascending aorta remains to the right of the main pulmonary artery, anterior to the right pulmonary artery and superior to the superior vena cava. The distal third of the ascending aorta lies anterior to the trachea and right main stem bronchus, courses superior to the pulmonary artery and posterior to the innominate vein. Sixty to 70% of aneurysm dissections occur in the ascending aorta (9).
The aortic arch curves upward between the ascending and descending aortas. Originating from the arch are the innominate (brachiocephalic) artery, the left common
carotid artery, and the left subclavian artery, which carry blood to the brain. Both the innominate and left common carotid arteries lie close to the anterior aspect of the trachea. The left subclavian artery lies to the left of the trachea.
carotid artery, and the left subclavian artery, which carry blood to the brain. Both the innominate and left common carotid arteries lie close to the anterior aspect of the trachea. The left subclavian artery lies to the left of the trachea.
The descending thoracic aorta lies distal to the left subclavian artery and curves posteriorly to lie posterior to the esophagus at approximately intercostal space 12. The thoracoabdominal aorta is the descending thoracic and the abdominal aorta.
The wall of the aorta is composed of the intimal, medial, and adventitial layers. The intimal surface is composed of a confluent monolayer of endothelial cells. It is responsible for the regulation of vasomotor tone, inflammatory and immunologic reactions and coagulation. Eighty percent of aortic wall thickness is comprised by the media, layers of smooth muscle cells intermeshed with connective tissue material. The adventitial layer contains the vasa vasorum, lymphatics and collagen (29).
TRANSESOPHAGEAL ECHO EXAMINATION OF THE THORACIC AORTA
Most segments of the thoracic aorta can be clearly imaged with multiplane transesophageal echocardiography (TEE) as the aorta descends along the esophagus. Two blind spots, the distal ascending aorta and the proximal aortic arch, occur due to the intervening trachea and left main stem bronchus. Epiaortic scanning may be useful during surgery in order to visualize these two areas.
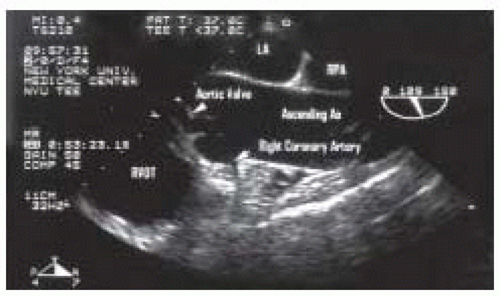
Examination of the ascending aorta begins with the mid esophageal aortic valve long axis view at 120 degrees (Fig. 31.2). Slight withdrawal of the probe and rotation of the angle back to 45 degrees will allow further visualization of the ascending aorta. The mid esophageal ascending aortic short axis view appears simply by rotating the angle between 0 to 45 degrees (Figs. 31.3 and 31.4). This view displays the main and right pulmonary arteries as longitudinal structures and the aorta as circular section. The diameter of the aorta may be measured in either the short- or long-axis views.
Examination of the descending thoracic aorta begins by turning the probe to the left from the midesophageal four-chamber view until a circular structure appears in the upper center of the echo image. This view is called the descending aorta short-axis view and is best viewed with an enlarged picture at a depth of 6 cm to 8 cm (Fig. 31.5). The rotation of the multiplane probe to 90 degrees will produce a horizontal long-axis view of the descending aorta (Fig. 31.6). The entire descending thoracic and upper
abdominal aorta can be imaged in either long- or short-axis by slowly advancing and withdrawing the probe. It is often difficult to pinpoint the exact location of a descending aortic lesion due to the changing positional relationship of the esophagus and the aorta. Localization of aortic wall lesions is often marked in terms of their distance from the left subclavian artery.
abdominal aorta can be imaged in either long- or short-axis by slowly advancing and withdrawing the probe. It is often difficult to pinpoint the exact location of a descending aortic lesion due to the changing positional relationship of the esophagus and the aorta. Localization of aortic wall lesions is often marked in terms of their distance from the left subclavian artery.
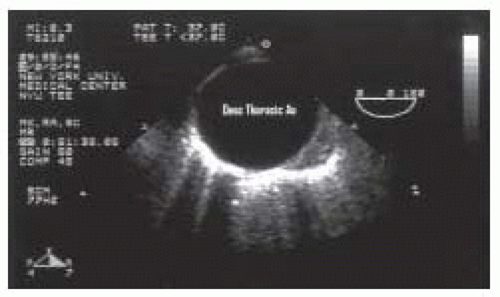 FIGURE 31.5. Transverse (00°) view of the descending thoracic aorta (Desc Thoracic Ao). From Konstadt |
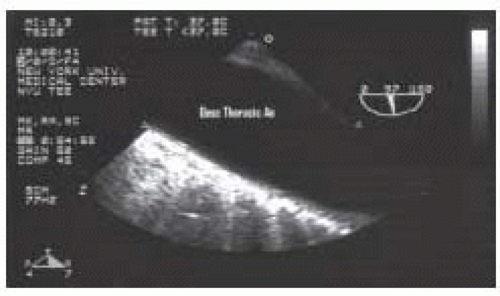 FIGURE 31.6. Longitudinal (970°) view of the descending thoracic aorta (Desc Thoracic Ao). From Konstadt |
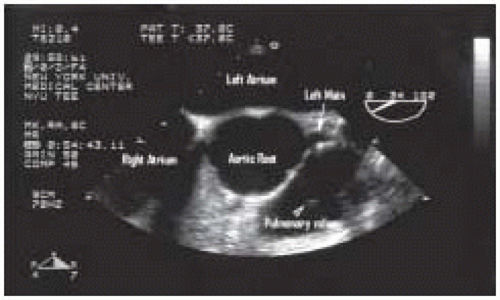
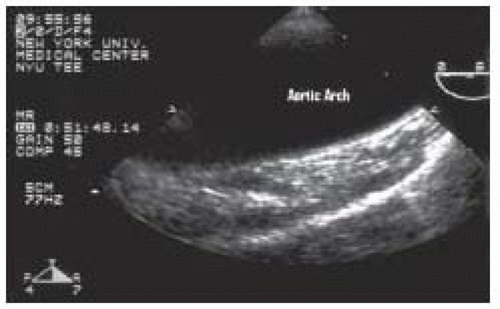
As the probe is withdrawn at an angle of 0 degrees the circular descending aorta becomes an elliptical structure, the aortic arch. This view is called the upperesophageal aortic arch long-axis view (Fig. 31.7). The distal arch is to the right of the image and the proximal arch to the left, with the posterior wall at the top and the anterior wall below. Turning the probe to the right and the left will allow full visualization of the length of the arch. A short-axis aortic arch view may be obtained by rotating the multiplane angle to 90 degrees (Fig. 31.8).
The arch vessels are often difficult to image adequately. Beginning with the upper
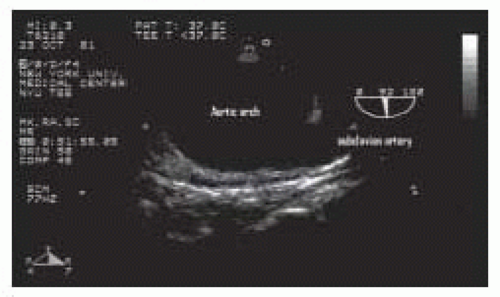
esophageal long-axis view, withdrawal of the probe superiorly, and rotation of the multiplane angle to 20 to 40 degrees may allow visualization of the proximal left subclavian and carotid arteries (Figs. 31.9, 31.10 and 31.11). Visualization of one of these two structures is reported to occur approximately 60% of the time, while both are visualized 75% of the time. The innominate artery and the distal ascending aorta are obscured from view by the air-filled trachea (30,31).
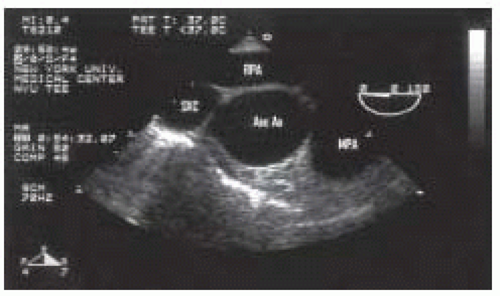
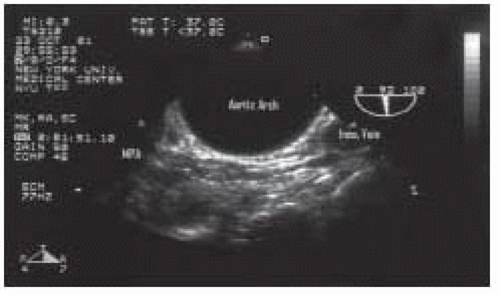 FIGURE 31.8. Longitudinal (920°) view of the mid aortic arch, demonstrating its relationship to the main pulmonary artery (MPA) and the innominate vein (Inno Vein). From Konstadt |
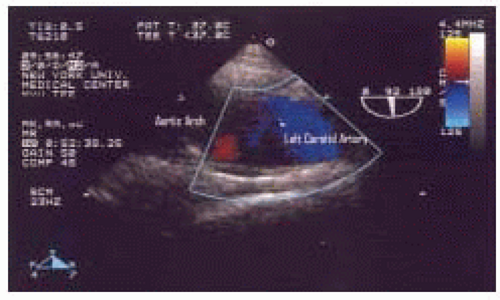
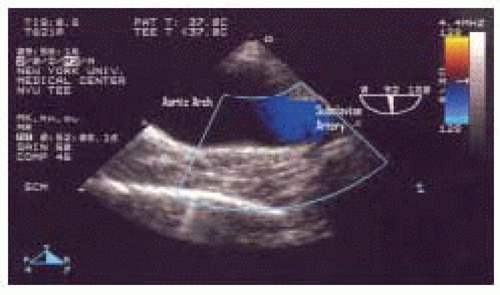 FIGURE 31.10. Color Flow Doppler in the longitudinal (920°) view of the mid aortic arch, demonstrating nonturbulent normal blood flow in the left subclavian artery. |
STRUCTURES
TEE Sensitivity and Specificity by Disease Classification
Comparison to Other Diagnostic and Monitoring Modalities
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree