Calculation of Stroke Volume
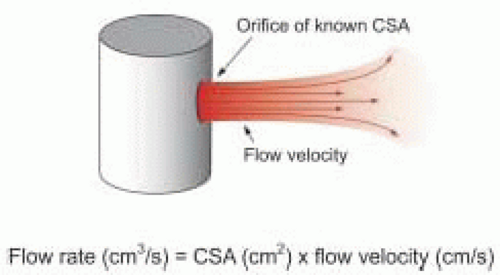
The flow rate of a fluid through a fixed orifice is directly proportional to the product of the cross-sectional area (CSA) of the orifice and the flow velocity of the fluid
within the orifice as given by the
hydraulic orifice formula (
Fig. 22.1):
Flow rate (cm 3/s) = CSA (cm2) × Flow velocity (cm/s)
Because the cardiovascular system is pulsatile, blood flow velocity varies. Nevertheless, the instantaneous flow rate of blood going through an orifice or blood vessel of constant cross-sectional area is directly proportional to the product of the cross-sectional area (CSA) of the orifice or blood vessel and the instantaneous blood flow velocity.
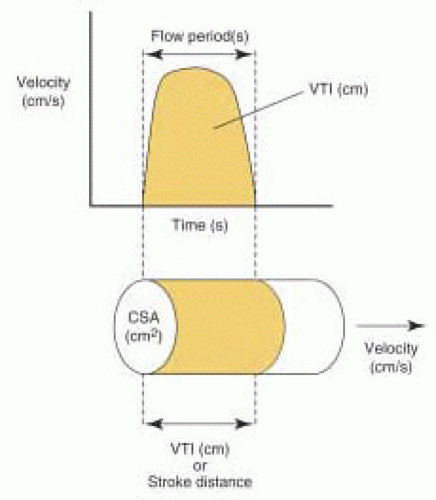
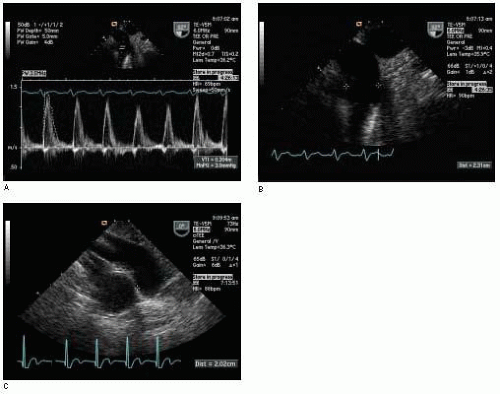
The acceleration and deceleration of blood flow velocity during the ejection period (or filling period) provides a distinct Doppler profile for a given orifice. The summation of velocities over the entire flow period is correctly called the
velocity-time integral (VTI), although it is also commonly referred to as the time-velocity integral (TVI). The VTI is equal to the area bounded by the Doppler flow velocity profile and the zero velocity baseline (
Fig. 22.2). It is measured by tracing the Doppler velocity signal using the calculation package built in the ultrasound machine. The VTI can be conceptually thought of as the distance that blood travels with each beat of the heart and is thus also called the stroke distance.
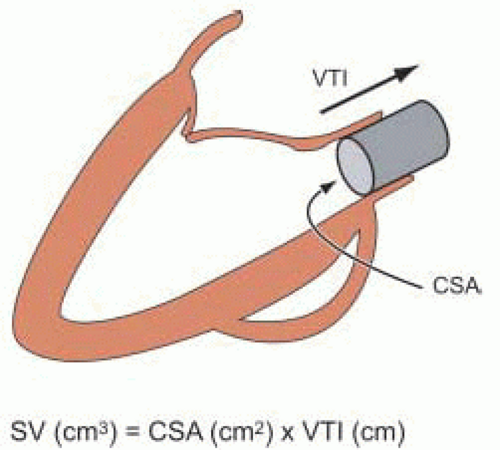
Stroke volume (SV) can be calculated as the product of the cross-sectional area and VTI (
Fig. 22.3):
SV (cm3) = CSA (cm2) × VTI (cm)
Stroke volume can be calculated at many different locations within the heart or great vessels by using the appropriate Doppler velocity signal to determine the VTI at the same location that 2-D imaging is used to determine cross-sectional area. Accordingly, the VTI is usually measured with pulse wave Doppler. However, continuous wave Doppler may be utilized to determine the aortic valve VTI in the absence of subvalvular or supravalvular aortic obstruction. In this case, the velocity signal obtained by continuous wave Doppler across the aortic valve should be the same as that obtained by pulse wave Doppler within the aortic valve.
Most often the cross-sectional area of the “orifice” to be measured is assumed to be circular and thus can be calculated using the formula for the area of a circle (of radius r) after measuring the orifice diameter (D) in cm:
CSA (cm2) = II × r2 = II × (D/2)2 = 0.785 × D2
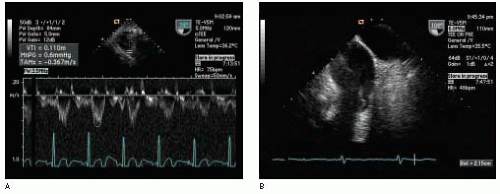
The Doppler method for determining stroke volume at a particular site is based on the following four assumptions, which are listed in
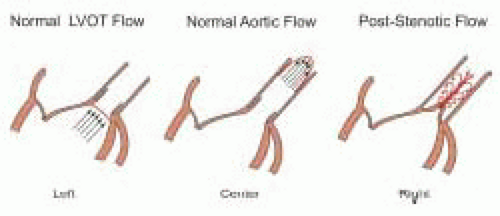
Table 22.2. First, blood flow is assumed to be laminar and the spatial flow velocity profile is assumed to be flat, as is generally the case in the LVOT (
Fig. 22.4). The narrow band of velocities and smooth spectral signal obtained with pulsed wave Doppler is evidence of laminar flow in the great vessels and across normal cardiac valves. A flat flow velocity profile can be demonstrated by showing uniform velocities while moving the pulsed wave Doppler sample volume from side to side within the flow of interest from two orthogonal views.
Second, cross-sectional area (i.e., diameter) and VTI measurements are assumed to be made at the same time and at the same anatomic location. Diameter is measured most accurately when the ultrasound beam is perpendicular to the blood-tissue interface, while VTI is measured most accurately when the ultrasound beam is parallel to blood flow. Thus, diameter measurements and Doppler velocity profiles are usually not recorded from the same imaging plane. Nevertheless, every effort should be made to perform these measurements at the same anatomic location and in close sequence in order to minimize error in the calculated stroke volume.
Third, the VTI used in calculating stroke volume is assumed to represent the average VTI. Therefore, several measurements should be averaged for a patient in normal sinus rhythm, whereas between 8 and 10 measurements should be averaged for a patient in atrial fibrillation, in order to most accurately estimate the average VTI.
Fourth, determination of cross-sectional area is assumed to be accurate. Changes in cross-sectional area during the flow period, or deviations from an assumed geometry (usually circular) are inherent problems in Doppler stroke volume calculations. Accurate determination of 2-D measurements for calculation of cross-sectional area is essential. In the case of an assumed circular orifice, a small error in diameter measurement will result in a large error in the calculated cross-sectional area due to the quadratic relationship between the radius and area of a circle (i.e., CSA = II × r2). The use of high frequency multiplane TEE probes for measuring diameters (or areas) undoubtedly increases the reliability of these measurements when compared to the use of lower frequency monoplane or biplane TEE probes.
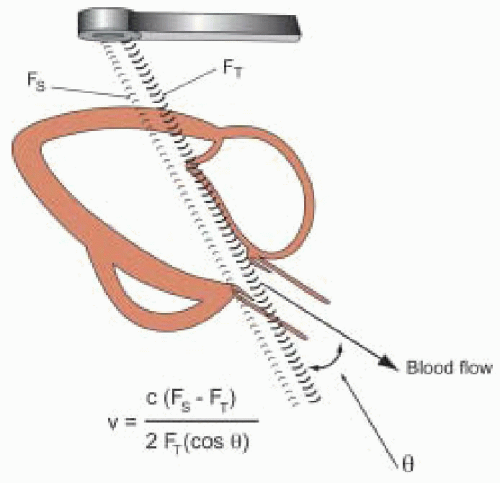
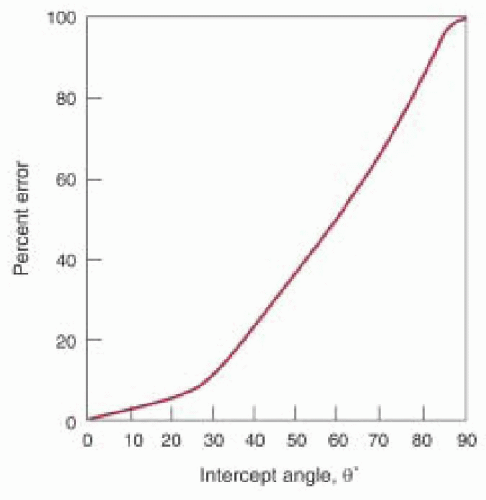
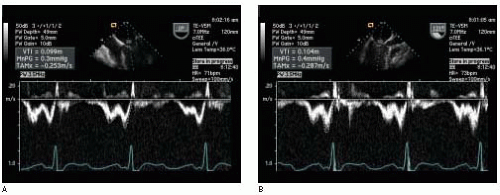
Fifth, the VTI is assumed to be recorded with the ultrasound beam parallel to the flow (i.e., the intercept angle θ = 0). In this case the velocities measured by Doppler are accurate based on a cosine θ = 1 in the Doppler equation (
Fig. 22.5). However, as θ increases from 20° to 60°, the error in the calculated Doppler velocity increases from 6% to 50% (
Fig. 22.6). Small adjustments in the TEE probe transducer position and the pulsed wave Doppler sample volume (or continuous wave Doppler beam) are necessary to obtain the highest velocity signal. Multiple imaging planes should be utilized when possible to ensure that the highest velocity signal is obtained. Use of the audio Doppler signal in addition to the visual Doppler display may aid the echocardiographer in optimal alignment of the ultrasound beam with the flow of interest. The highest velocity signal obtained (the loudest audio signal) will correlate with the most parallel alignment
of the Doppler beam with blood flow. While underestimation of velocities is always a potential source of error in Doppler stroke volume determination with TEE imaging, it is undoubtedly less of a concern with multiplane than with monoplane or biplane TEE imaging.
Calculation of Cardiac Output
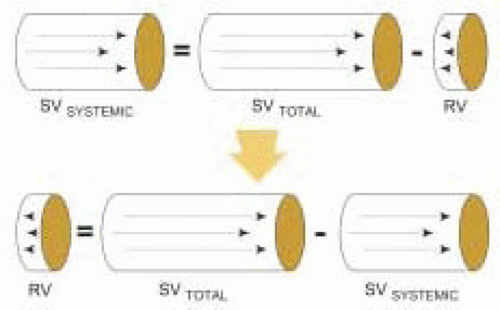
Cardiac output (CO) can be estimated with 2-D Doppler after determining a Doppler stroke volume and measuring heart rate (
5). Cardiac output is calculated as the product of stroke volume and heart rate (HR). Cardiac index (CI) is calculated by dividing cardiac output by body surface area (BSA):
CO (l/min) = SV (cm3) × (1 liter / 1000 cm3) × HR (bpm) CI (l/min/m2) = CO (l/min) / BSA (m2)
Cardiac output measurements performed with TEE, usually measured at the LVOT or aortic valve in the absence of aortic regurgitation, have been shown to correlate well with measurements made by thermodilution (
6,
7,
8,
9,
10,
11). An accurate estimation of cardiac output depends on accurate determinations of the VTI and cross-sectional area. It is advisable to interrogate from multiple windows when possible. Accuracy is improved by assessing multiple Doppler flow profiles, typically 3-5 for a regular rhythm and 10 for an irregular rhythm. Furthermore, accuracy is improved if multiple diameter measurements are averaged prior to calculation of cross-sectional area as any error in this measurement is squared as previously discussed.
Stroke volume calculations for estimation of cardiac output are preferably made with multiplane TEE at the LVOT or aortic valve for three reasons. First, the acceleration of blood through the LVOT or aortic valve during systole favors laminar flow with a flat flow velocity profile, in contrast to the parabolic flow velocity profile present in the ascending aorta or pulmonary artery. Doppler determination of the VTI within a small sample of the cross-sectional area will be more representative of that throughout the cross-sectional area when the flow velocity profile is flat. Second, multiplane TEE provides excellent views of the LVOT and aortic valve for accurate determinations of LVOT diameter and aortic valve cross-sectional area. Third, the LVOT is more circular and changes shape very little during the cardiac cycle when compared to the main pulmonary artery or mitral valve. Measurements made at the main pulmonary artery or mitral valve are less reliable than those made at the LVOT and aortic valve (
12). Although the cross-sectional area of the aortic valve orifice changes dramatically throughout systole, the cross-sectional area of the aortic valve during midsystole can be used to provide a good estimate of transaortic stroke volume by Doppler.
Data for Transaortic Valve Stroke Volume Calculation
(Fig. 22.8)The
continuous wave Doppler beam is placed through the aortic valve from either the transgastric long-axis view or the deep transgastric long-axis view for determination of the VTI
AV. The cross-sectional area of the aortic valve can be determined by one of two methods. Planemetry can be used to measure the area (cm
2) of the aortic valve orifice
during midsystole from a cine of the midesophageal short-axis view of the aortic valve (
9).
Alternatively, a cine of a “normal” aortic valve from the same midesophageal short-axis view is used to measure the side (S) in cm of the equilateral opening of the valve during midsystole. Several measurements may be made and then averaged in order to improve accuracy. The formula for the area of an equilateral triangle is then used to calculate the cross-sectional area of the aortic valve:
CSA AV (cm2) = 0.433 × (S)2
The RVOT may be visualized using a transgastric RV inflow-outflow view with the transducer rotated from 110° to 150° and the probe turned to the right. The pulsed wave Doppler sample volume is placed in the RVOT just proximal to the pulmonic valve for determination of the VTIRVOT. The diameter (cm) of the RVOT is best obtained from the same view at the same location for determination of cross-sectional area, using the formula for the area of a circle:
CSARVOT (cm2) = 0.785 × DRVOT2
Alternatively, the diameter (cm) of the RVOT can be measured from the upperesophageal short-axis view of the aortic arch in some patients.
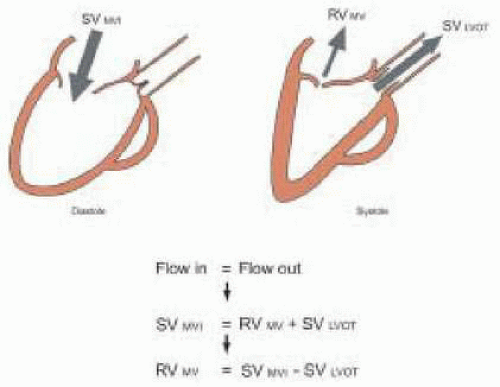
The pulsed wave Doppler sample volume is placed at the level of the mitral valve annulus, using the midesophageal four-chamber view (alternatively, the midesophageal two-chamber view or midesophageal long-axis view may be used) for determination of the VTI
MV. While the mitral valve orifice is not truly elliptical during diastole, it is more elliptical than circular. The American Society of Echocardiography concluded in its document on quantitation of Doppler echocardiography that assumption of a circular orifice has generally worked well for all valves other than the tricuspid (
13). Nevertheless, it may be preferable to estimate the cross-sectional area of the mitral valve, using the formula for an ellipse. The long and short diameters (cm) of the mitral valve annulus can be approximated using measurements from the midesophageal four-chamber and two-chamber views. The formula for an ellipse can then be used to calculate the cross-sectional area of the mitral valve:
CSA MV (cm2) = 0.785 × D1 × D2
The irregular semielliptical shape of the mitral valve orifice and the fluctuation in its size during diastole make stroke volume measurement at this location less reliable than at the LVOT or aortic valve (
12).