During stress examinations in which low or high doses of dobutamine are administered, monitoring the patient within the magnet is mandatory. In general, monitoring during a CVMR examination requires that the same precautions be taken and the same emergency equipment be available as in any other stress examination. A physician trained in cardiovascular emergencies and resuscitation must be at the
scanner. Apart from specific contraindications to CVMR such as the presence of retro-orbital metal, cerebral clips, or non-MRI compatible pacemakers, the contraindications are identical to those for stress echocardiography and are listed in
Table 16.4.
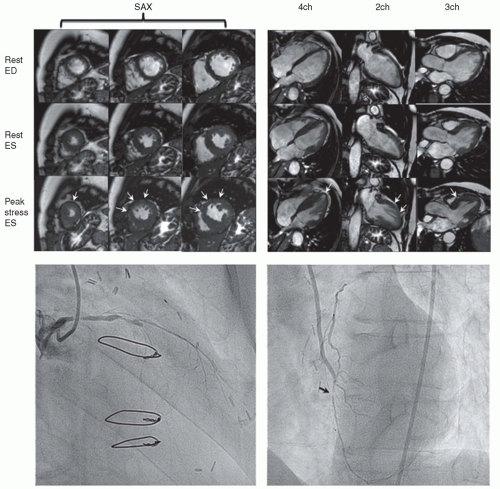
In a routine clinical setting, DSMR proved to be feasible and safe, and it resulted in a high number of diagnostic ex aminations (89.5%) in patients without contraindications to MRI (
10). Wahl et al. (
10) reported a 5-year experience of high-dose DSMR in 1,000 consecutive patients and showed a safety profile virtually identical to that previously reported for dobutamine stress echocardiography. One patient suffered sustained ventricular tachycardia and emergent defibrillation was carried out, while no cases of death or myocardial in farction occurred over the years (
Table 16.6). Approximately 50% of the patients had inducible ischemia during stress CVMR, thereby closely reflecting the clinical practice in a tertiary center. Data from the large Euro CVMR registry has
confirmed the low periprocedural complication rate of highdose DSMR (
11). Thus, although adverse events are rare, the staff must be prepared to remove the patient from the magnet rapidly if necessary and must comply closely with the test termination criteria (
Table 16.3). Whereas in most other modalities, “eye-to-eye” contact between patient and examiner takes place, communication during CVMR is usually established via a microphone system and video cameras, although it can also be conducted personally. This does not hinder the safety process if the patient is monitored carefully for symptoms, blood pressure changes, and wall-motion abnormalities (
Table 16.2). Either standard equipment can be placed outside the scanner room and connected to the patient with special extensions through a waveguide in the radiofrequency cage or special CVMR-compatible equipment, available at many CVMR sites, can be used. A defibrillator and all medications needed for emergency treatment must be available at the CVMR site. A specific problem associated with monitoring within the magnet is that of assessing ST-segment changes from the ECG. However, because wallmotion abnormalities precede ST changes and because such abnormalities can be readily detected with fast CVMR, monitoring is effective without a diagnostic ECG and can also be performed in patients with left-bundle branch block, who are routinely evaluated with dobutamine stress echocardiography despite nondiagnostic ST segments. Such wall-motion abnormalities can be detected immediately after image reconstruction, which is completed 5 to 10 seconds after image acquisition. In addition, real-time imaging permits the immediate detection of wall-motion abnormalities and can be used for monitoring (
12). Such real-time acquisition has been found to yield a similar diagnostic accuracy for the detection of inducible wall-motion abnormalities (sensitivity and specificity for real-time MR imaging 81% and 83%) when compared to conventional MR cine imaging (
13). However, spatial resolution is less, and thus we recommend ECG-triggered breathhold imaging as the basis for the final diagnosis.