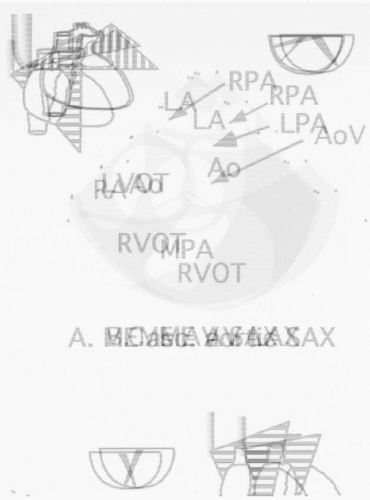
should follow those suggested by the American Society of Echocardiography (7) and ASE/SCA guidelines (8). Appropriate TEE views for evaluation of congenital heart lesions are described in Table 20.1 and Fig. 20.1. Assessment of the connections of the various cardiac segments, atrial arrangement or situs, venoatrial, atrioventricular, and ventriculoarterial connections should be performed. Septal and valvar structures should then be evaluated, including assessment of flow velocities with Doppler echocardiography. In general, the approach for each lesion is to define the intracardiac anatomy and associated defects, assess the ventricular function, and evaluate any residual lesions (Table 20.2). Comprehensive reference atlases include Pediatric Echocardiography, edited by N. Silverman (Williams and Wilkins), Transesophageal Echocardiography in Congenital Heart Disease, edited by O. Stumper and G. Sutherland (Little, Brown and Company), and Congenital Heart Disease in Adults, edited by J. K. Perloff and J. S. Child (W. B. Saunders).
TABLE 20.1. Congenital Heart Lesions—Appropriate TEE Views for Evaluation of Congenital Heart Lesions | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
to one-third of all lesions, occurring more commonly in women (9). There are four types of atrial septal defects:
TABLE 20.2. Indications for Primary Surgery, Common Postoperative Complications and Indications for Reoperation in Congenital Heart Disease | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
of arrhythmias, which are generally related to preoperative atrial dilatation or postoperative incisional reentry (16). Operative patch closure is generally recommended if the shunt is large with a pulmonary blood flow-to-systemic blood flow ratio of 1.8:1 or higher and right ventricular enlargement. Percutaneous closure with a variety of devices is becoming increasingly available and can be considered in adults. Patients with smaller shunts have a lower incidence of congestive heart failure, pulmonary hypertension, and arrhythmias but are at risk for paradoxical embolization. In patients with ostium primum defects, surgical valve repair with or without annuloplasty may reduce the severity of the mitral and tricuspid regurgitation. If severe mitral regurgitation persists, valve re-repair or replacement is necessary.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree