is nonspecific. All patients with dilated cardiomyopathy should have their immediate family members screened for the disease because of the high incidence of familial dilated cardiomyopathy (3). Goerss and associates revealed that at least 24% of patients with dilated cardiomyopathy have familial disease and that there appears to be no demonstrable differences in clinical or pathological findings between familial and nonfamilial disease, other than confirmed family history (3). Clinical presentation is usually with heart failure, which is often progressive. Arrhythmias, thromboembolism, and sudden death are common and may occur at any stage. End-diastolic and end-systolic dimensions and volumes are typically increased and all variables of systolic function, such as ejection fraction, stroke volume, and cardiac output are uniformly decreased (Fig. 33.1) (2). While left ventricular mass is uniformly increased, wall thickness varies among patients and typically is within normal limits. Ventricular contractility is usually globally reduced, yet superimposed regional wall motion abnormalities can also be present if substantial coronary artery disease exists. These similar findings occur despite the wide variety of etiologies of dilated cardiomyopathy. Other two-dimensional echocardiographic features of dilated cardiomyopathy include dilation of the mitral valve annulus, leading to incomplete coaption of the anterior and posterior leaflets, causing functional mitral insufficiency, enlarged left and/or right atrial chambers, and apical ventricular thrombi (Fig. 33.2).
TABLE 33.1. Classification of Cardiomyopathies | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
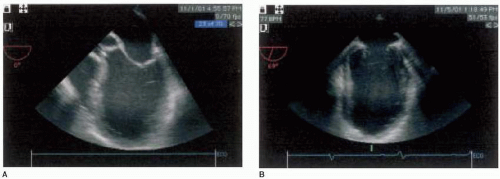 FIGURE 33.1. Midesophageal four-chamber view (A) and midesophageal mitral commissural view (B) of dilated cardiomyopathy, demonstrating increased ventricular dimensions. |
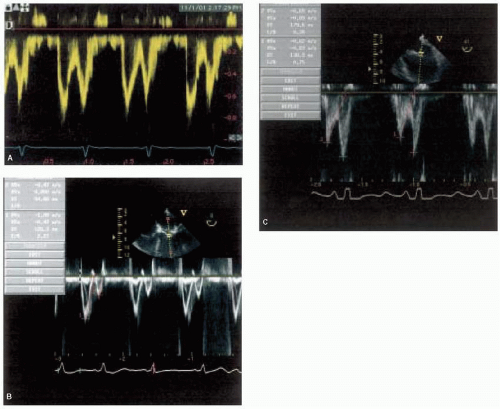
(decreased E/A ratio) inflow profile. When patients begin to decompensate, stroke volume and cardiac output decrease and a restrictive physiology (increased E/A ratio) inflow profile predominates because of decreased left ventricular compliance and increased left ventricular filling pressures (5). St. Goar and associates evaluated patients with idiopathic-dilated cardiomyopathy and severe heart failure who were undergoing cardiac catheterization during evaluation for heart transplantation (5). Simultaneous echocardiographic evaluation revealed that the transmitral flow velocity pattern was characterized by normal peak early filling velocity, low normal isovolumic relaxation time, shortened acceleration and deceleration times of early diastolic flow, decreased early flow velocity integral, and absent or decreased filling during atrial contraction (5). This pattern reflects interaction between elevated transmitral driving pressure and the compromised relaxation and compliance of a left ventricle, functioning on an elevated pressure-volume curve (5). The evolution of diastolic dysfunction from abnormal relaxation to restrictive physiology has been reliably reproduced in animal models of dilated cardiomyopathy (6). Ohno and associates measured left ventricular and left atrial pressures and left ventricular volume, and calculated left ventricular and left atrial stiffness during the development of congestive heart failure produced by rapid pacing in awake, unsedated dogs (6). They revealed that early in congestive heart failure, slowing left ventricular relaxation reduced the maximal early diastolic left atrial-left ventricular pressure gradient, decreasing the peak early filling rate (6). As congestive heart failure progressed, this was overcome by an increase in left atrial pressure that augmented the early diastolic left atrial-left ventricular pressure gradient, increasing peak early filling rate (6). Increasing left ventricular stiffness during the development of congestive heart failure progressively shortened the early filling deceleration time and augmented the early filling deceleration rate (6). These observations suggest that the early filling deceleration time reflects left ventricular stiffness (6). Clinical studies indicate that, of the wide variety of variables derived from mitral inflow velocity profile, deceleration time has the most prognostic value because shorter deceleration time (restrictive physiology) portends a worse prognosis (7,8). Rihal and associates examined the clinical and echocardiographic characteristics of patients with the clinical diagnosis of dilated cardiomyopathy to determine the prognostic implications of these characteristics (7). Patients with severe congestive heart failure had lower indices of systolic function and were more likely to have significant mitral regurgitation and greater left atrial and right ventricular dilation (7). Left ventricular diastolic filling abnormalities were prominent and independently associated with severe symptoms, with a restrictive-type filling pattern (increased E/A ratio, short deceleration time) being common (7). Xie and associates confirmed that patients with congestive heart failure with poor prognosis can be identified by a restrictive transmitral flow pattern, female gender, and advanced functional class (8). Of these, the restrictive transmitral flow pattern appears to be the single best predictor of mortality over two years (8). In patients with dilated cardiomyopathy, the deceleration time increases, and the mitral inflow velocity profile becomes less characteristic of restrictive physiology as congestive heart failure symptoms are treated with medication. Improvement in these indices of diastolic dysfunction is associated with a high probability of clinical improvement and survival (9,10). Pinamonti and associates evaluated patients with dilated cardiomyopathy at presentation and after three months of medical treatment (9). They found that persistence of restrictive filling at three months was associated with a high mortality and transplantation rate, whereas patients with reversible restrictive filling had a high probability of clinical improvement and excellent survival (9). Similarly, a retrospective analysis by Lee and associates revealed that, in patients with congestive heart failure, the initial restrictive diastolic filling pattern can be altered to a nonrestrictive filling pattern with medical therapy and a change in diastolic filling to a nonrestrictive pattern is associated with improved survival (10). Another useful prognostic indicator in patients with dilated cardiomyopathy is the status of pulmonary artery pressure as estimated from tricuspid regurgitation velocity (11). Abramson and associates evaluated patients with dilated cardiomyopathy and found that patients exhibiting a high velocity of tricuspid regurgitation (> 2.5 meters/second) had more hospitalizations for congestive heart failure and a higher mortality rate when compared to patients exhibiting a lower velocity of tricuspid regurgitation (< 2.5 meters/second) (11). Peak velocity of tricuspid regurgitation was the only prognostic variable that predicted overall mortality, mortality due to myocardial failure, and hospitalization for congestive heart failure (11). Higher tricuspid regurgitation velocities are usually seen in patients with dilated cardiomyopathy and a restrictive physiology mitral inflow velocity profile. The presence of a restrictive physiology mitral inflow velocity profile and high velocity tricuspid regurgitation identifies patients with dilated cardiomyopathy who are at increased risk for development of heart failure and death.
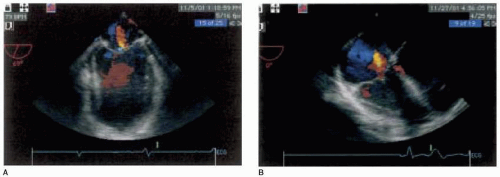 FIGURE 33.2. Midesophageal four-chamber view of dilated cardiomyopathy, with color flow Doppler, demonstrating functional mitral insufficiency (A) and functional tricuspid insufficiency (B). |
phenotypic manifestations, even in a single family cohort with the same molecular genetic defect. The extent of hypertrophy at any given site can vary greatly and bears importantly on the manifestations of the disease. Mutations in sarcomeric contractile protein genes cause the cardiomyopathy. Typical morphological myocardial changes include myocyte hypertrophy and disarray surrounding areas of increased loose connective tissue. Abnormal thickening of coronary arteries may also occur. Arrhythmias and premature sudden death are common.
to determine the severity of the gradient. If pressure gradients of greater than 30 mm Hg at rest are present, the potential for further hypertrophy and deterioration is highly likely (13). Obstruction of the left ventricular outflow tract may cause the aortic valve to close early (premature midsystolic closure). Systolic anterior motion of the mitral valve apparatus distorts mitral valve configuration, resulting in mitral insufficiency. Thus, varying degrees of mitral insufficiency almost invariably accompany the obstructive form of hypertrophic cardiomyopathy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree