Assessment of Cardiac Masses
Kirk Spencer
The identification of intracardiac masses is an important use of echocardiography. TEE provides an unparalleled window into the heart. TEE allows improved definition of mass size, shape, and mobility compared to the transthoracic window (1,2,3,4,5,6,7,8,9). The higher frequency and proximity of TEE probes provide superior evaluation of the tissue characterization of a mass, which can be useful in differential formulation. In addition, clarification of mass location, attachment, extent, and myocardial involvement are easily accomplished with TEE (5,7,10,11,12). The benefit of TEE is particularly strong for the evaluation of masses in the LAA, RA, and aorta or in those patients with technically limited transthoracic images (1,7,9). Three-dimensional reconstruction from the TEE window allows dynamic presentation of views simulating intraoperative visualization of cardiac masses (13,14).
Cardiac surgery for intracardiac masses is an indication for intraoperative TEE that has been recognized (15). Although patients may have had preoperative TTE and even TEE exams, a complete intraoperative evaluation is paramount for confirmation of the mass prior to surgical exploration (15,16,17). Likewise, rapid and accurate interpretation after the discovery of an unsuspected cardiac mass in the operating room is essential (18,19,20). Once a cardiac mass is confirmed, intraoperative TEE is useful for guiding the surgical approach for removal (10, 21,22,23). In addition, placement of surgical cannula in the presence of cardiac masses, whether in the IVC, SVC, or aorta, may be guided using TEE (17,24). Intraoperative TEE to assess the hemodynamic effects of a cardiac mass can prove invaluable. Lastly, intraoperative TEE allows assessment of the surgical intervention. Intraoperative TEE permits evaluation for residual mass as well as sequelae of mass removal on valvular and myocardial function prior to leaving the operating room (15).
The value of intraoperative TEE during surgery for cardiac masses was highlighted in a recent series (17). In this study, 9 in 75 patients had their surgical course altered by intraoperative transesophageal echocardiography. Six patients had the operative procedure altered due to the detection of additional pathology. This included one patient with a right atrial myxoma in whom an unknown LA myxoma was detected. One subject with an RA mass had alteration of the caval cannulation site. One intraoperative TEE failed to confirm a LA thrombus and surgery was cancelled. Three patients went back on cardiac bypass for valve repair after mass removal based on information provided by the intraoperative TEE.
APPROACH/STRUCTURES
Equally important as complete knowledge of pathologic masses within the heart is an understanding of the normal structures, anatomic variants, and artifacts encountered during a transesophageal examination that may be confused with pathologic findings. Detailed knowledge of these normal findings prevents undo concern or delay in the operative setting to pursue further evaluation or solicit opinions regarding a structure, which is, in fact, a normal variant. Correct interpretation of normal intracardiac masses may in fact prevent surgical evaluation or inappropriate medical therapy for these structures. Patients referred for surgical removal of intracardiac masses have been spared operation by a thorough and knowledgeable intraoperative transesophageal echocardiographic examination. Though some of the structures detailed in the paragraphs below may seem, to an experienced transesophageal echocardiographer, as unlikely to be mistaken for a pathologic finding, all of these structures have been confused with pathology at one time or another (3,8).
IVC/SVC/RIGHT ATRIUM
The right atrium and vena cava can be imaged in a midesophageal bicaval view, which displays a long axis of the vena cava as it enters the right atrial cavity. In this view,
the eustachian valve is noted at the juncture of the inferior vena cava and right atrium. This structure projects into the right atrial cavity and can be mistaken for an intracardiac mass. There is wide variation in eustachian valves among patients and although typically thin and mobile, eustachian valves may be thick and rigid. It is the typical location of the eustachian valve that allows it to be discerned from a pathologic structure.
the eustachian valve is noted at the juncture of the inferior vena cava and right atrium. This structure projects into the right atrial cavity and can be mistaken for an intracardiac mass. There is wide variation in eustachian valves among patients and although typically thin and mobile, eustachian valves may be thick and rigid. It is the typical location of the eustachian valve that allows it to be discerned from a pathologic structure.
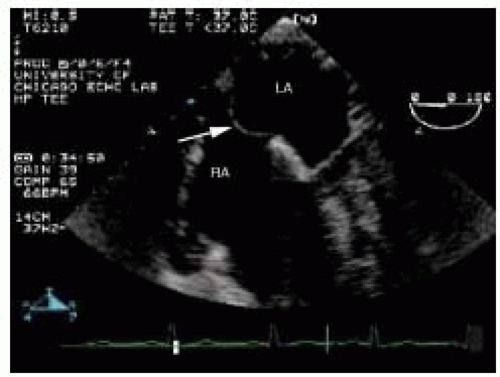
One might also see a Chiari network in the RA. This structure consists of a meshwork of fibers originating about the margin of the eustachian and thebesian valves and attaches in the area of the crista terminalis. This network represents the vestigial remains of the right valvulae venosae and septum spurium that fail to resorb into the wall of the RA (25). Echocardiographically, this structure appears as a thin mobile filamentous echodensity within the RA cavity (Fig. 19.1) (26,27). The anatomic variation in Chiari networks is quite wide because many patients have no visible Chiari network and others have prominent ones. Distinction of this structure from an intracardiac mass is made by its typical echocardiographic appearance and by noting, that although the body is mobile, this structure is tethered at its margins. These remnants are seldom clinically important, but have been reported as sources of entrapment for emboli and right heart catheters (28,29).
Within the body of the right atrium itself, several structures are noteworthy for potential confusion with pathologic masses. The right atrial wall is trabeculated with pectinate muscles that must be noted as normal variants. Pectinate muscles are discerned by their perpendicular alignment from the right atrial wall and their regular spacing from one another. When the SVC is viewed in long axis, there is a prominent ridge at the juncture of the SVC and the right atrium, the crista terminalis. There is somewhat wide anatomic variation in this ridge and it may appear quite prominent in some patients. Its location adjacent to the right atrial appendage can sometimes be confused with a right atrial appendage thrombus.
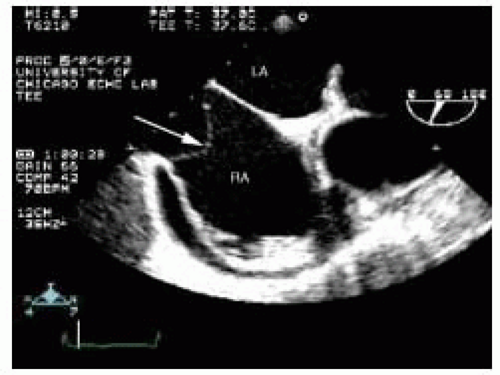 FIGURE 19.1. This linear right atrial mass (RA) is a Chiari network, which is a normal variant. LA, left atrium. |
When there is a loculated pericardial effusion behind the right atrium, the right atrial wall may appear to be an intracardiac linear density. This abnormality can be readily discerned with the injection of agitated saline, which delineates the right atrial cavity. The tricuspid annulus may accumulate fat and be prominent. Particularly if imaged off-axis, this may appear to represent an intracardiac mass between the RA and RV. This can readily be discerned by imaging in multiple planes and noting smooth continuity with the rest of the cardiac structures (Fig. 19.2).
Two normal anatomic variants of the interatrial septum can also be confused with intracardiac masses, the first of which is lipomatous hypertrophy. Pathologically, lipomatous hypertrophy represents a benign proliferation in which mature adipocytes infiltrate the interatrial septum. This fatty involvement is more prominent at the cephalad and caudal ends sparing the fossa ovalis, giving the interatrial septum a dumb-bell shaped appearance (Fig. 19.3). Fatty infiltration of the interatrial septum is denoted not only by its typical pattern of involvement, but also by the hyperrefractile nature of the fatty deposits that may cast a prominent acoustic shadow. Although this disorder represents a spectrum of interatrial septal thickening, diagnostic thickness criteria of 1.5-2.0 cm have been proposed (30,31,32,33). At the other end of the spectrum, lipomatous hypertrophy can reach massive proportions and infiltrations several centimeters in diameter have been reported (34,35).
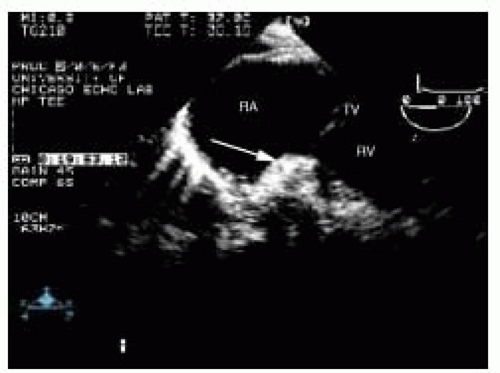 FIGURE 19.2. A view of the right atrium (RA), tricuspid valve (TV), and right ventricle (RV) showing a prominent tricuspid annulus (arrow), which can be confused with an intracardiac mass. |
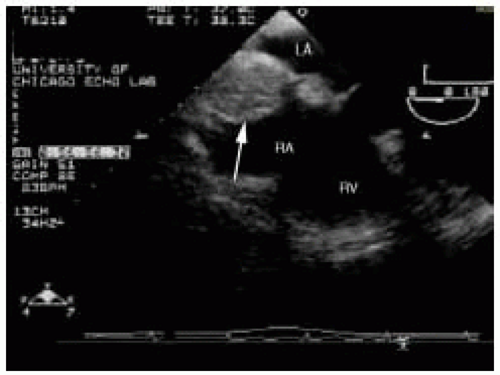 FIGURE 19.3. Interatrial septum with lipomatous hypertrophy (arrow). Note the sparing of the fossa ovalis. RA, right atrium; LA, left atrium; RV, right ventricle. |
An interatrial septal aneurysm, which has a prevalence of between 2% and 10%, is another anatomic variant that can mimic an intracardiac mass (36,37,38). The definitions of redundant septum and aneurysmal septum are somewhat arbitrary. Published criteria define an interatrial septal, aneurysm as a redundancy of at least 1.5 cm of the septum, with a displacement past the midline of at least 1 cm (36,39). In some patients the redundancy of the interatrial septum is prominent with excursions of several centimeters into the left or right atrium depending on interatrial pressures (Fig. 19.4). When imaging off-axis, the aneurysmal portion of the septum may protrude into and out of plane giving the appearance of a mobile interatrial mass. This may present during intraoperative transesophageal echocardiography as an unexpected mass, despite a preoperative transthoracic echocardiogram, because of TEE’s superior rate of detection for this lesion (37,39).
RIGHT VENTRICLE/PULMONARY ARTERY
There are several normal structures within the right ventricle and right ventricular outflow track that can be confused with pathologic intracardiac masses. Within the right ventricular chamber itself, the primary structures to beware of are the ventricular trabeculations. The right ventricle is a heavily trabeculated chamber and in the setting of right ventricular pressure overload, these trabeculations may become quite prominent. Even in the normal right ventricle, there is a prominent muscular band, the moderator band, which is a normal finding. Catheters within the right ventricle must of course be discerned from intracardiac masses. Although this would seem straightforward, because catheters are curvilinear structures being interrogated with a flat imaging plane, only small portions of the catheter may be visualized at any one time. This may give the appearance of small mobile intracardiac echodensities. In addition, because catheters are echodense, they are prone to forming reverberation and side lobe artifacts, which must not be mistaken for pathologic structures.
Within the pulmonary artery itself, the primary consideration is not confusing ultrasound clutter for an intraluminal mass. The main pulmonary artery is typically imaged in the midesophageal RV inflow-outflow view. This puts the pulmonary artery in the far field where, because of ultrasound beam spreading, there is often prominent clutter. With adjustment of the ultrasound imaging controls, such as gain and focus, as well as manipulating the probe itself this clutter artifact can usually be “cleaned up.” Clutter artifact is also prominent in near field. When the right pulmonary artery is being imaged from the midesophageal ascending aortic short-axis view, there is often prominent clutter within the right pulmonary artery. Again, attention to focus and gain can minimize this artifact and avoid the false interpretation of a right pulmonary artery thrombus.
PULMONARY VEINS/LEFT ATRIUM/LEFT ATRIAL APPENDAGE
A normal structure that can be confused with an intracardiac mass in the LA is the fold of tissue between the left upper pulmonary vein and the LA proper. This ridge has anatomic variation and at times can be prominent. In
addition, though this ridge is typically linear, the end of the ridge may appear somewhat bulbous giving it a “Q-tip” appearance. This can be confused with an LA or LAA thrombus. Another structure that can lead to the erroneous diagnosis of an LAA mass is the transverse sinus. When there is fluid in this pericardial reflection it may appear to be continuous with the LAA cavity. The LAA wall may then appear to be a linear mass. In addition, this structure often has echodense fibrinous material in it that can be confused with an LAA thrombus.
addition, though this ridge is typically linear, the end of the ridge may appear somewhat bulbous giving it a “Q-tip” appearance. This can be confused with an LA or LAA thrombus. Another structure that can lead to the erroneous diagnosis of an LAA mass is the transverse sinus. When there is fluid in this pericardial reflection it may appear to be continuous with the LAA cavity. The LAA wall may then appear to be a linear mass. In addition, this structure often has echodense fibrinous material in it that can be confused with an LAA thrombus.
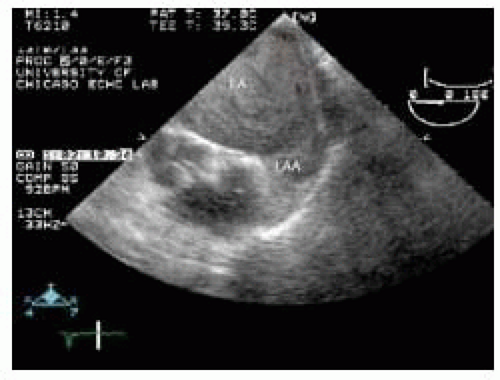
The left atrial appendage is a structure that commonly has features suggesting an intracardiac mass. The left atrial appendage has pectinate muscles that may simulate left atrial appendage thrombus. Pectinate muscles typically have a tissue characterization more similar to muscle than thrombus. In addition, the pectinate muscles typically emanate perpendicular from the left atrial appendage wall and appear multiple in a somewhat regularly spaced pattern. A multiplane approach to the evaluation of left atrial appendage is essential. The LAA often has multiple lobes (40) and the tissue separating these infoldings may be confused with thrombus.
Another phenomenon, although not truly a normal anatomic finding but nevertheless doesn’t represent an intracardiac mass, is left atrial spontaneous echo contrast or “smoke.” Spontaneous echo contrast in the left atrial chamber is noted by its echodense swirling pattern and may be confused with left atrial thrombus. Within the left atrial appendage, particularly in situations of low appendage flow such as atrial fibrillation, dense spontaneous echo contrast can be very difficult to discern from intraappendage thrombus (Fig. 19.5). Confirming the presence of a thrombus in multiple planes can be helpful to prevent misinterpretations.
LEFT VENTRICLE
Several normal structures within the left ventricular cavity, when imaged along tangential planes, can lead to the false diagnosis of an intracardiac mass. The LV papillary muscles are usually easily discerned from pathologic masses by their location and attachments to the submitral apparatus, as well as having a tissue characterization that is similar to that of the myocardium. When imaging from a midesophageal four-chamber view, the base of the interventricular septum may appear prominent, suggesting an intramyocardial mass. However, far more commonly is the presence of sigmoid septum. This is a normal variant of aging in which remodeling of the left ventricular cavity projects the basilar interventricular septum into the left ventricular outflow tract.
The left ventricular cavity can also have thin linear structures, which cross the chamber particularly near the left ventricular apex. These false chords are common normal anatomic variants. Lastly, the left ventricular apex is often foreshortened and difficult to completely image from a midesophageal four-chamber view. This foreshortening can give the appearance that the apex is filled in and thus lead to the erroneous conclusion of an apical thrombus. When evaluating the left ventricular apex from the transesophageal approach, care must be taken to retroflex the probe to fully elongate the left ventricular cavity. Additional imaging from the transgastric long-axis and reverse four-chamber views may be helpful.
AORTA
False positive masses in the thoracic aorta are primarily ultrasound artifacts. As the aorta is in the near field when imaging from the esophagus, near field clutter is common and must be distinguished from intraaortic thrombus or atheroma. This is done by noting that the aorta can be moved independent of the clutter and that the clutter often extends outside the borders of the aorta. Linear artifacts can be noted in the descending or ascending aorta. If not appreciated as artifacts these can lead to erroneous conclusions that have significant clinical implications. The mechanism of these artifacts has been elucidated and distinction from true pathology is often possible (41).
PATHOLOGY
Most pathologic cardiac masses represent thrombus, infection, or tumor. A transesophageal echocardiographic examination cannot definitively diagnose an intracardiac mass, as this requires histologic confirmation. However, a reasonably secure diagnosis can usually be made by incorporating
the echocardiographic location and appearance of the mass with available clinical data. In addition, there may be associated echocardiographic findings that substantially alter the differential diagnostic choices for an intracardiac mass.
the echocardiographic location and appearance of the mass with available clinical data. In addition, there may be associated echocardiographic findings that substantially alter the differential diagnostic choices for an intracardiac mass.
Cardiac involvement by malignancy may be primary (origin within the heart) or secondary/metastatic (origin distant from heart). Metastatic neoplasms are far more common than are primary tumors by a 20-30:1 ratio (42,43,44). Of metastatic involvement of the heart, the most common tumors are lung, breast, and lymphoma/leukemia followed by esophageal and uterine (42,43,44). Cardiac involvement may occur from direct invasion or metastatic spread from distant disease. Certain tumors, such as melanoma, have a predilection for metastasizing to the heart. Although most secondary involvement of the heart by malignancy is pericardial (˜75%), it is the myocardial and intracardiac involvement this chapter focuses on, because these present as cardiac masses.
Intraoperative TEE evaluation of metastatic disease of the heart is not simply an imaging exercise. Patients with metastatic tumor of the heart who have made it to the operating room may be there for attempt at surgical cure. In this case, careful TEE evaluation of tumor extent and myocardial infiltration is crucial. If intraoperative and preoperative tumor staging differ, the surgical approach may be aborted or altered (45). In addition, patients may be undergoing palliative surgery to relieve the hemodynamic effects of tumor involvement (for example, intracardiac obstruction). Intraoperative evaluation of hemodynamic improvement after tumor resection is vital to operative success.
Primary cardiac tumors are less commonly found with an incidence of 0.1%-0.3% in autopsies series (42,44,46). Most (75%-80%) are benign; more than one-half of these are myxomas. Less common cardiac tumors include rhabdomyoma, fibroma, and hemangioma. Primary malignant cardiac tumors are almost exclusively types of sarcoma (> 95%) (47,48,49). Of sarcomas, the angiosarcoma and rhabdomyosarcoma are the most common (50). Those tumors suitable for primary resection can be evaluated with intraoperative TEE to guide surgical therapy.
Cardiac involvement by tumors may be asymptomatic or cause significant morbidity, such as pericardial tamponade, arrhythmias, or congestive heart failure. The lack of malignant potential does not always indicate a “benign” clinical course because nonmalignant intracavitary tumors may produce obstruction to blood flow or partially dislodge, causing embolization.
IVC/SVC/RIGHT ATRIUM
The most common intracardiac mass in the vena cava and right atrium is a thrombus. Right atrial thrombi occur in several settings. Thrombi from the deep venous system may embolize and become entrapped in the right atrium (51). This clinical scenario likely happens far more frequently than detected as most thromboemboli rapidly pass through the right atrium. Thromboemboli originally from peripheral veins appear as mobile serpiginous irregular masses in the right atrium that do not have a point of attachment to the right atrial wall. These thrombi may become entrapped in the foramen ovale, which is a mechanism for paradoxical embolization (1,52).
Another cause of right atrial thrombus is in situ formation, which occurs in conditions with low atrial blood flow, such as right atrial dilatation in cardiomyopathic states and atrial fibrillation. In situ thrombi typically are homogenous echodensities adherent to the right atrial wall with a broad base of attachment. Although right atrial thrombi have been reported along the interatrial septum, this is unusual. Right atrial thrombi commonly have mobile irregularities extending from their surface. These projections may be large enough to prolapse into the RV cavity during diastole (Fig. 19.6).
Right atrial thrombi associated with atrial fibrillation typically form on the right atrial free wall or within the right atrial appendage (Fig. 19.7). In patients with atrial fibrillation, approximately 15% of cardiac thrombi are located within the right atrial appendage. Right atrial appendage thrombi are best visualized in a midesophageal bicaval view. Careful movement of the probe into and out of the esophagus is required to interrogate the entire body of the right atrial appendage.
A special case of in situ right atrial thrombus formation is that associated with intracardiac catheters. Superior vena caval catheters, particularly hemodialysis
catheters that are stiff and have higher flows, may lead to endocardial damage that serves as a nidus for mural thrombus formation. Given the orientation of the vena cava and right atrium, superior vena caval catheter-associated thrombi most commonly form near the inferior vena cava—right atrial junction, often on or near the eustachian valve (Fig. 19.8). Right atrial thrombi in this location and others, associated with patients with chronic renal failure, typically are more inhomogeneous and partially calcified.
catheters that are stiff and have higher flows, may lead to endocardial damage that serves as a nidus for mural thrombus formation. Given the orientation of the vena cava and right atrium, superior vena caval catheter-associated thrombi most commonly form near the inferior vena cava—right atrial junction, often on or near the eustachian valve (Fig. 19.8). Right atrial thrombi in this location and others, associated with patients with chronic renal failure, typically are more inhomogeneous and partially calcified.
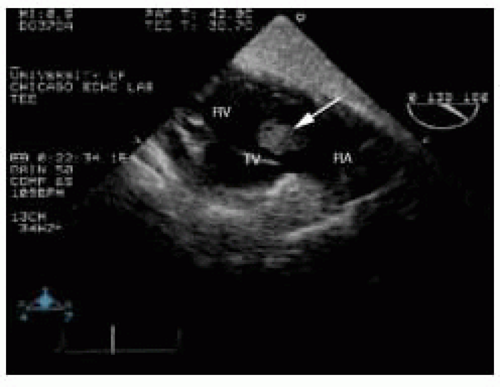 FIGURE 19.6. Transgastric long-axis view of the tricuspid valve (TV), showing a large right atrial thrombus (arrow) prolapsing through the tricuspid valve. RA, right atrium; RV, right ventricle. |
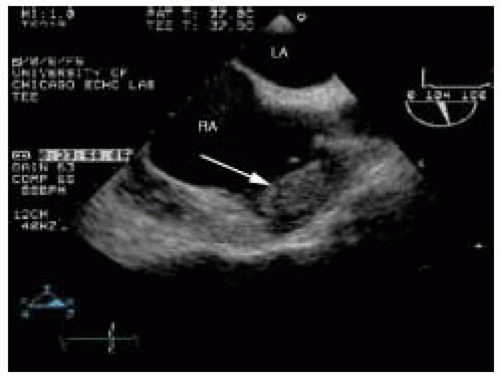 FIGURE 19.7. Large thrombus in the right atrial appendage (arrow). RA, right atrium; LA, left atrium.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|