CRITICAL ISSUES ADDRESSED BY INTRAOPERATIVE ECHOCARDIOGRAPHY IN PATIENTS UNDERGOING PROCEDURES FOR CONGESTIVE HEART FAILURE
Assessment of Left and Right Ventricular Function
Assessment of left ventricular (LV) and right ventricular (RV) function is critical to the proper evaluation and perioperative management of patients with end-stage CHF. Using IOE can provide excellent qualitative and quantitative information on RV and LV function.
Dilated CMP is characterized by dilation of both ventricular and atrial chambers associated with systolic heart failure (
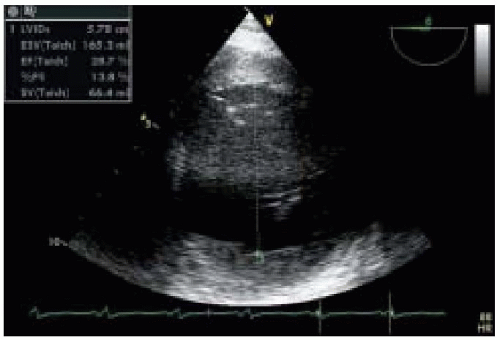
9). Both motion mode (M-mode) and two-dimensional (2-D) echocardiography can be used for the evaluation of LV systolic function. The high time resolution of M-mode echocardiography enables it to accurately measure ventricular internal dimension and wall thickness. Additionally, placing the M-mode beam at the mitral chordal level to obtain a transgastric longitudinal view of the LV makes it possible to compute LV fractional shortening,
a rough measurement of LV systolic function with a normal range of 25% to 45%, using the following formula:
Fractional shortening (%) = (LVIDd − LVIDs)/LVIDd × 100%
(LVIDd = left ventricular internal diameter at enddiastole; LVIDs = left ventricular internal diameter at end-systole).
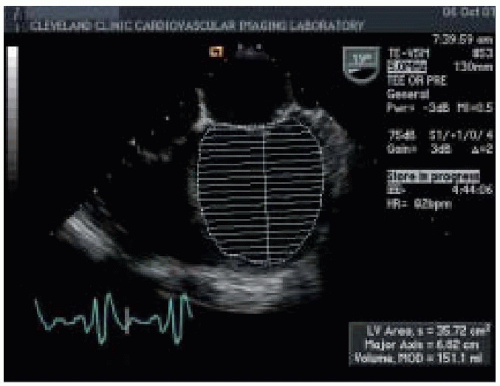
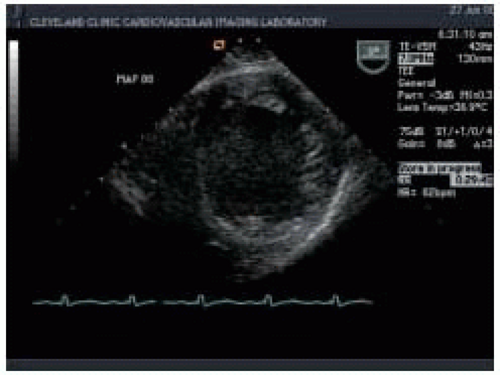
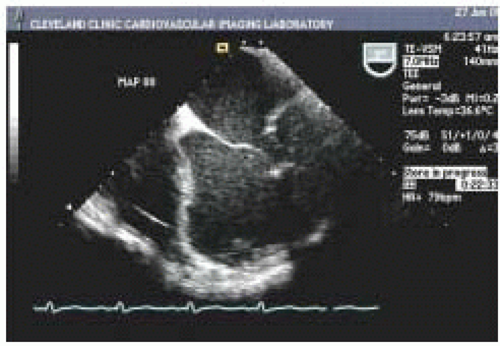
Two-dimensional echocardiography provides both qualitative and quantitative evaluation of systolic ventricular function. Midesophageal four-chamber (ME 4-chamber) and two-chamber (ME 2-chamber) views and the midesophageal long-axis (ME LAX) view, transgastric short-axis (TG SAX) views (basal, midpapillary, and apical), and the transgastric long-axis view (TG LAX) allow assessment of both global and regional ventricular function. Two-dimensional quantitative measures of LV systolic function include ventricular dimensions, volume, stroke volume (SV), cardiac output (CO), ejection fraction (EF), and regional wall motion abnormalities (
Figs. 35.4 and
35.5). Left ventricular ejection fraction (LVEF) can be calculated from LV end-systolic volume (LVESV) and end-diastolic volume (LVEDV) as the ratio (LVEDV-LVESV)/LVEDV. Cardiac output can be calculated using the area-length formula across a cardiac valve (most commonly the aortic valve):
CO = 0.785 × LVOT D2 × LVOTVTI × HR
(LVOT = left ventricular outflow tract; D = LVOT diameter; LVOTVTI = velocity time integral, measured with Doppler spectrum across the aortic valve; HR = heart rate). IOE determination of CO is helpful, even when a pulmonary artery catheter is used, because some patients with dilated CMP present with some degree of tricuspid valve regurgitation, which renders the thermodilution technique inaccurate.
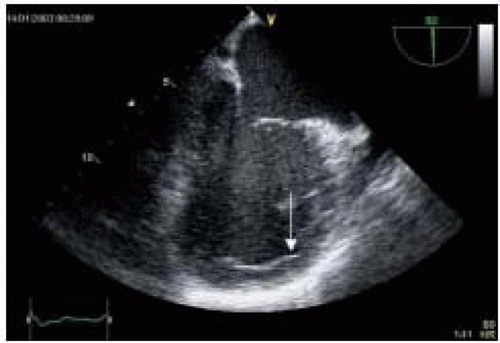
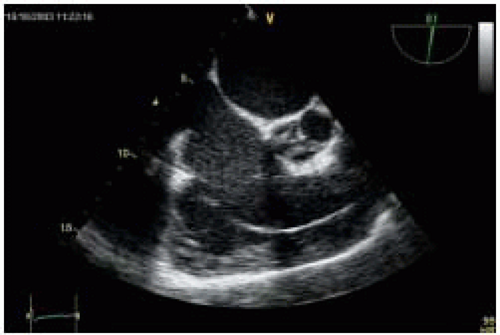
In addition, patients with end-stage CHF often present with coexisting pathology such as acquired left ventricle aneurysm (LVA). LVA is the final expression of transmural infarct expansion and is often accompanied by anterior infarction in the area of the distribution of the left anterior descending coronary artery (
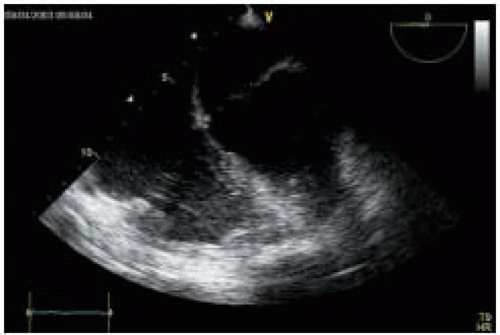
Fig. 35.6). Thrombi are frequently detected within aneurysms and are often associated with systemic embolization (
Fig. 35.7). Therefore,
careful echocardiographic assessment is mandatory if cardiopulmonary bypass is to be successful.
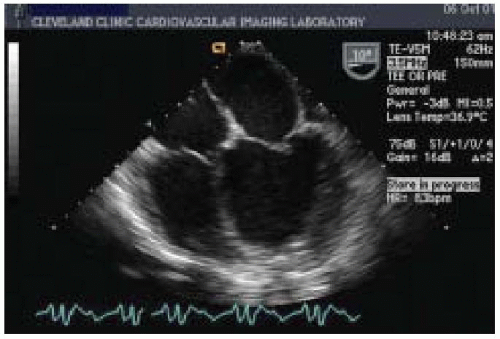
Evaluation of RV systolic function is a critical part of the intraoperative management of patients with end-stage CHF. Preexisting pathologic conditions in the RV, such as ischemia, infarction, CMP, and pulmonary hypertension (PHTN) are major risk factors for RV failure. Sometimes, the extent of RV dysfunction becomes apparent only when a sudden increase in venous return challenges an already impaired RV. With dilated CMP, there is an increase in systolic ventricular interaction, making RV systolic performance more dependent on the LV to generate pressure (
10). Under these circumstances, sudden LV unloading may depress RV function further.
Two-dimensional/M-mode echocardiography can provide several quantitative and qualitative measurements of RV systolic function. Wall thicknesses over 0.5 cm as measured by M-mode echocardiography are considered abnormal and suggest elevated pulmonary artery pressure, pulmonary valve stenosis, or infitrative CMP. The standard 2-D transesophageal echocardiographic (TEE) views used to evaluate RV function are the ME 4-chamber view, the ME RV inflow-outflow view, the transgastric mid-short-axis (TG mid-SAX) view, and the transgastric right ventricular inflow (TG RV inflow) view. Quantitative assessment of RV function with automated border detection in the midesophageal four-chamber view has been used to calculate RV fractional area change (RVFAC) (
11):
RVFAC = (end-diastolic area) − (end-systolic area/end-diastolic area) × 100%
Signs of RV dysfunction include hypokinesis or akinesis of the RV free wall, and RV dilation caused by volume or pressure overload. Normally, the RV end-diastolic cross-sectional area is less than 60% of the LV end-diastolic cross-sectional area. With dilatation, however, the RV changes its shape from triangular to round, with concomitant enlargement of the right ventricular outflow tract (RVOT) and flattening of the bulge in the interventricular septum from right to left can be seen in the ME 4-chamber view and the ME RV inflow-outflow view (
Figs. 35.8 and
35.9). With RV pressure overload, a maximal leftward septal shift is noted at end-systole, whereas RV volume overload is associated with a maximum reversed septal curvature in mid-diastole. As RV dilation becomes moderate or severe, the RV replaces the LV in forming the cardiac apex in the ME 4-chamber view, and the end-diastolic cross-sectional area of the RV may equal or exceed that of the LV. Tricuspid annular plane systolic excursion (TAPSE) may decrease to less than 20 mm, accompanied by impaired RV systolic function (
12). At this stage, pulsed wave Doppler (PWD) evaluation of blood flow in the hepatic vein may reveal attenuation of the systolic inflow wave.
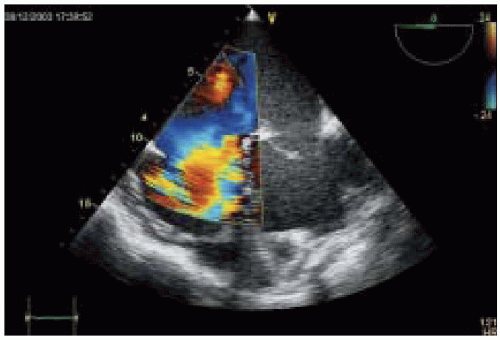
Nonetheless, pulmonary artery (PA) pressure estimates remain one of the most important quantitative measures of RV systolic function. As RV dysfunction progresses, dilation of tricuspid valve (TV) annulus occurs, causing varying degrees of tricuspid regurgitation (
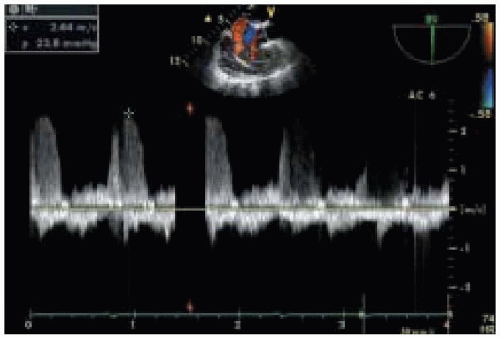
Fig. 35.10). Measuring the velocity and pressure gradient of the tricuspid regurgitant jet (
Fig. 35.11) reveals the gradient between RV and right atrial (RA) pressure and, when added to an estimate of RA pressure (transduced from the central venous line), allows calculation of RV systolic pressure
(
Table 35.5). Right ventricular SV and CO can be measured directly by imaging the RVOT tract and PA in the upper esophageal aortic arch short-axis (UE aortic arch SAX) view (
Fig. 35.12). By measuring the diameter (D) of PA annulus and using PWD mode to determine velocity time integral (VTI) across pulmonic valve, right ventricular SV, and CO can be calculated using the formulas SV = PA D2 × 0.785 × PA
VTI and CO = SV × HR.
Growing evidence suggests that both systolic and diastolic dysfunction play important roles in the clinical presentation and prognosis of patients with end-stage CHF. Patients with hypertrophic, infiltrative, or primary restrictive
CMP may report symptoms of heart failure despite normal EF, a condition known as diastolic heart failure (
13,
14). In addition, in patients with preexisting systolic dysfunction, abnormalities in diastolic dysfunction may be significantly related to the severity of cardiac symptoms and prognosis in patients with CHF (
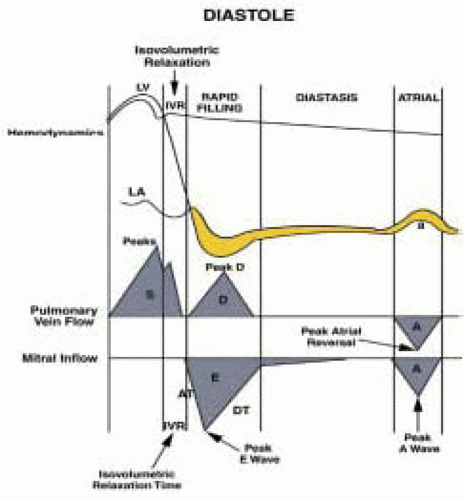
15). Diastole is the interval between aortic valve closure and mitral valve closure and can be divided into four phases:
1. Isovolumic relaxation
2. Early rapid diastolic filling
3. Diastasis
4. Late diastolic filling caused by atrial contraction (
Fig. 35.13).
Parameters of diastolic function include ventricular relaxation, myocardial compliance, and chamber compliance. Ventricular relaxation is measured by isovolumic relaxation time (IVRT), the rate of pressure decline (dP/dT), and time constant of relaxation (τ). Myocardial compliance is estimated by the ratio of change in volume to change in pressure (dV/dP). Chamber compliance is assessed by measuring early diastolic LV filling (E wave), deceleration time (DT), late diastolic LV filling (AM wave), the AP wave (reversed) of LA contraction, the s wave of the systolic LA filling phase, and the d wave of the LA diastolic filling phase.
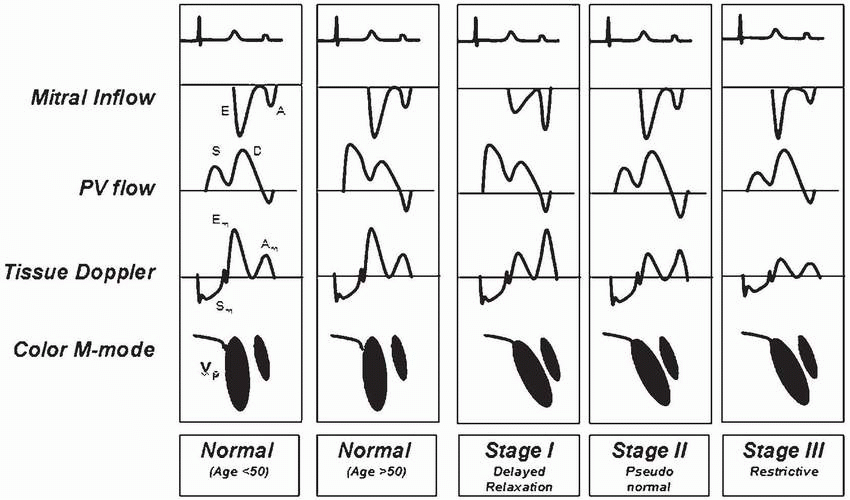
The earliest stage of abnormal diastolic filling is
impaired relaxation with inverse E/A ratio as the major Doppler abnormality (
Table 35.6). With progression of the disease to moderate diastolic dysfunction,
pseudonormalization of diastolic filling flow occurs because of impaired myocardial relaxation balanced by elevation of mean LA pressures (
Table 35.6). The diagnosis is confirmed by abnormal PV flow or response to Valsalva maneuver. The
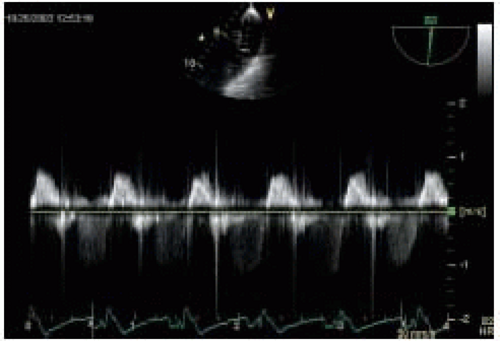
restrictive filling pattern is the most advanced form of diastolic dysfunction that can be associated with either normal or abnormal systolic function. It may accompany advanced infiltrative CMP, such as amyloidosis, advanced hypertensive disease, or dilated CMP. The hallmark of the disease is elevated LA pressures with increased LV stiffness that causes a large E wave, short DT, a small AM wave, and a very small s/d ratio on PV PWD trace (
Figs. 35.14 and
35.15) (
Table 35.7) (
16).
Surgical Procedure-Related Issues
Surgical approaches to end-stage CHF are rapidly becoming more numerous and sophisticated. Substantial advances have been made in myocardial revascularization, mitral valvuloplasty, ventricular remodeling (i.e., ECPP and LV partial resection), ventricular constraint techniques (i.e., Acorn CorCap external splinting, Myosplint internal splinting), mechanical ventricular support, TAH, and heart transplantation. During the period before cardiopulmonary bypass (CBP) is initiated, IOE focuses on the evaluation of
1. RV function, severity of TR, and the potential need for RV mechanical support
2. Baseline LV function
3. Potential sequelae of end-stage-dilated cardiomyopathy, such as LVA, LA mural thrombi, MR, and diastolic dysfunction
4. Potential right-to-left shunting through PFO, which may contribute to hypoxemia
5. The presence of AR or mitral stenosis (MS)
6. The presence of ascending aortic atheroma, which necessitates changing the cannulation strategy and myocardial protection.
This information guides the development of CPB strategy. At the time of separation from CPB, IOE is useful for evaluating the adequacy of deairing of the cardiac chambers. It is also important at that time to establish a comprehensive baseline study for future comparison and to assess LV and RV filling patterns, native valve function, the quality of the valve repair, and the presence of any intracardiac shunts. Given the importance of RV function in the outcome of the procedure, an objective qualitative assessment of RV systolic and diastolic function by RVFAC,
RV end-diastolic volume (RVEDV), RV dP/dT, TV annulus diameter, and severity of TR is important. These parameters will allow comparison between baseline and postoperative RV performance and dictate postoperative management in the event of acute deterioration of RV function, which is often seen when respiratory insufficiency occurs.