Assessment in Aortic Valve Surgery
Christopher A. Troianos
Lori Heller
Maged Argalious
Intraoperative echocardiography during aortic valve surgery provides critical information for surgical decision-making and hemodynamic management. Transesophageal echocardiography (TEE) provides high-resolution images of the aortic valve due to the close proximity of the valve to the esophagus. Evaluation of leaflet morphology and mobility, degree of calcification, aortic root disease, and etiology of valve dysfunction are important aspects of two-dimensional evaluation. Accurate determination of valve and aortic root dimensions is important for guiding therapy and choosing the type and size of a prosthesis to implant. Postoperative assessment identifies complications associated with repair or replacement, and prompts surgical intervention to correct inadequate valve repair and reoperation for complications. Clinical information provided by TEE permits appropriate hemodynamic management for patients with aortic valve disease, which is particularly important in patients with manifestation of chronic disease, whether they have stenosis, regurgitation, or both. The application of Doppler echocardiography (pulsed wave, continuous wave, and color), with two-dimensional imaging allows for the complete evaluation of stenotic and regurgitant lesions (discussed in detail in Chapter 15, Assessment of the Aortic Valve). This current chapter reviews the transesophageal echocardiographic anatomy, aortic valve disease pathophysiology, and echocardiographic evaluation of the aortic valve, with particular emphasis on critical issues that arise during aortic valve surgery. Indications for evaluation, implications for surgical intervention, and associated cardiovascular lesions are also discussed.
CRITICAL ISSUES DURING AORTIC VALVE SURGERY
Echocardiography provides essential information in the evaluation of patients with aortic valve disease. Intraoperative echocardiography is used to confirm the preoperative diagnosis, determine the feasibility of repair versus replacement, measure the size of valve to be implanted, and evaluate the implanted or repaired valve for complications. Preoperative valve sizing is important when valves of limited availability, such as homografts, are to be implanted (1). For patients undergoing aortic valve replacement for aortic stenosis, intraoperative TEE has been shown to alter the surgical plan in 13% of patients (Table 30.1) (2). The patient with mild to moderate aortic valve disease scheduled for nonaortic valve cardiac surgery, as well as the patient with previously unsuspected aortic valve disease present clinical dilemmas. TEE assists with this intraoperative decision making. The size of the annulus must be considered when deciding whether a valve with only moderate stenosis should be replaced. Patients with small annular diameters may not derive as significant a benefit from aortic valve replacement as patients in whom a larger valve could be implanted. The issue of whether or not to intervene in patients with mild to moderate disease is more important
for patients with stenosis rather than regurgitation, because of the higher mortality associated with reoperation in patients with aortic stenosis who have had previous coronary artery bypass grafting (CABG) (3). Finally, intraoperative echocardiography is used to differentiate aortic valve disease from other causes that produce a gradient between the left ventricle and aorta, such as hypertrophic obstructive cardiomyopathy and supravalvular stenosis.
for patients with stenosis rather than regurgitation, because of the higher mortality associated with reoperation in patients with aortic stenosis who have had previous coronary artery bypass grafting (CABG) (3). Finally, intraoperative echocardiography is used to differentiate aortic valve disease from other causes that produce a gradient between the left ventricle and aorta, such as hypertrophic obstructive cardiomyopathy and supravalvular stenosis.
TABLE 30.1. Impact of Intraoperative TEE during Aortic Valve Replacement in 383 Patients (2) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||
Role of TEE in Surgical Decision-Making
Intraoperative TEE among patients with known aortic valve disease undergoing valve replacement is used to confirm the preoperative diagnosis and determine the etiology of valve dysfunction. High-resolution images, owing to the close proximity of the valve and the esophagus, permit accurate diagnosis of the mechanism of valve dysfunction, a key aspect for determining the feasibility of repair versus replacement. The vast majority of aortic valves suitable for repair have regurgitant lesions rather than stenotic lesions. Valve repair for patients with aortic dissection involves resuspension of the cusps, and is easily performed and highly successful in the absence of additional leaflet pathology.
Postoperatively, TEE is used to evaluate the success of repair or function of the prosthetic valve. The degree of residual aortic regurgitation is an important aspect of valve repair evaluation and determines the need for further surgery and possible valve replacement. The number and area of regurgitant jets present after aortic valve replacement are less than after mitral valve replacement, but there is a similar percentage decrease of regurgitant jet area after protamine administration (4). Patients undergoing the Ross procedure for autograft replacement of their aortic valve also require evaluation of the prosthetic pulmonic valve.
TEE is important for guiding hemodynamic therapy during aortic valve surgery. An accurate evaluation of left ventricular function is important during the immediate postoperative period because of the inherently low ventricular compliance present among patients with left ventricular hypertrophy, due to long-standing aortic stenosis or chronic hypertension. Left ventricular volume is more accurately determined by two-dimensional echocardiographic assessment of LV cross-sectional area than by filling pressures measured with a pulmonary artery catheter (4a). Characteristically, patients with low LV compliance often require volume infusion despite high filling pressures in the postbypass period. Clinical information provided by TEE permits appropriate hemodynamic management for patients with aortic valve disease before, during, and after aortic valve surgery.
APPROACH
Focused Intraoperative Echocardiography Exam
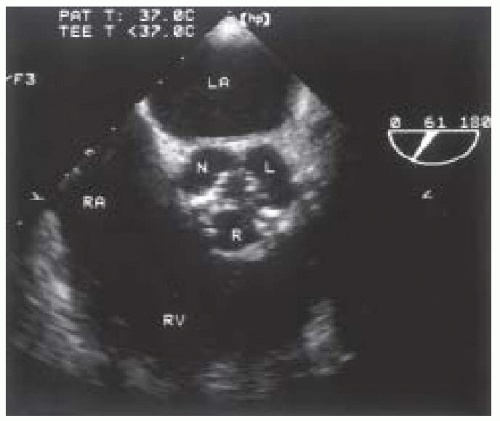
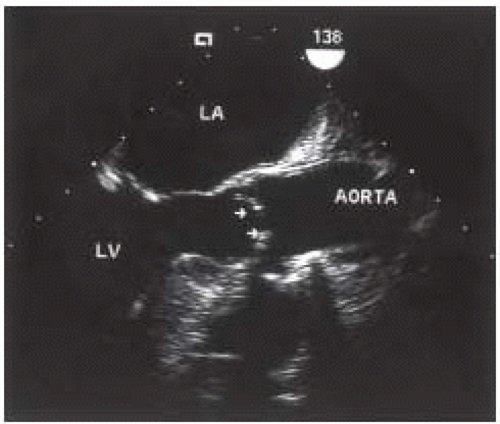
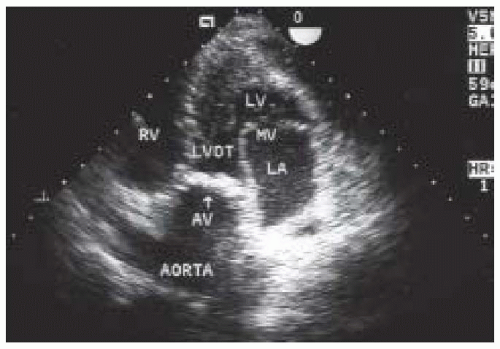
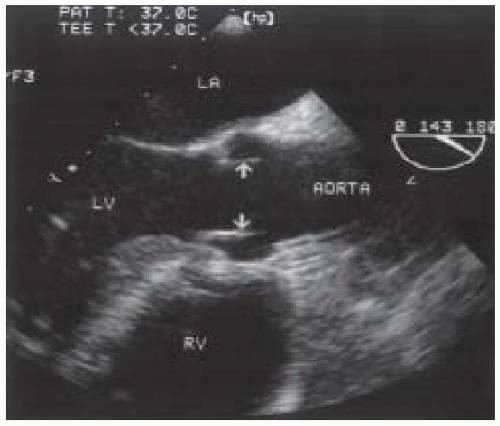
A focused intraoperative echocardiography exam is a brief pointed assessment on the anatomy and function most pertinent to the surgeon. For patients in whom the preoperative diagnosis of aortic valve disease is confirmed and well established, this usually entails a verification of the preoperative findings including the elucidation of the etiology of valve dysfunction, an estimation of valve size, and an assessment of associated findings. For patients with severe aortic stenosis, the diagnosis is confirmed simply by a two-dimensional echocardiographic evaluation of the valve, utilizing the midesophageal aortic valve short-axis view (Fig. 30.1). The valve appears severely restricted and heavily calcified. This aortic valve short-axis view is developed at the midesophageal level by rotating the transducer forward 30° to 60° and anteflexing the probe. This view is important for tracing the aortic valve orifice area, using planimetry, and for identifying the site of aortic regurgitation using color flow Doppler. Rotating the multiplane angle forward to 110° to 150° develops the midesophageal aortic valve long-axis (ME AV LAX) view (Fig. 30.2). This view provides imaging of the left ventricular outflow tract, aortic valve, and aortic root and allows differentiation of valvular from subvalvular and supravalvular pathology.
Quantifying the severity of stenosis by either gradient or area determination is usually not necessary during the focused exam of a patient whose diagnosis of aortic stenosis was firmly established preoperatively, but is often included in a more comprehensive exam. Area determination by planimetry in patients with severe aortic stenosis may be difficult to perform due to shadowing of the valve by the heavy calcification. Area determination with the continuity equation in patients with severe stenosis is of benefit to the novice echocardiographer who requires practice in obtaining the transgastric views necessary for measuring aortic valve flow. A peak velocity measurement provides some indication of the severity of stenosis, but must be taken in the context of the left ventricular function.
A focused exam in patients with severe aortic regurgitation undergoing aortic valve surgery involves a two-dimensional echocardiographic evaluation to determine the etiology of the regurgitation (leaflets vs. aortic root) and a color flow Doppler interrogation of the left ventricular outflow tract to evaluate the severity of the regurgitation. The etiology directs the surgeon as to the feasibility of repair versus replacement, and the techniques to be employed for repair. The postoperative assessment is important for ascertaining the success of the repair. The degree of residual aortic regurgitation is an essential aspect of valve repair evaluation and determines the need for further revisions or possible valve replacement.
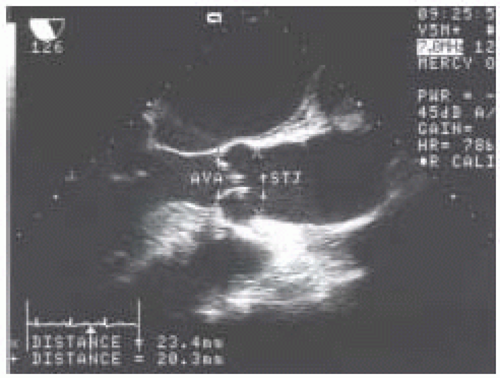
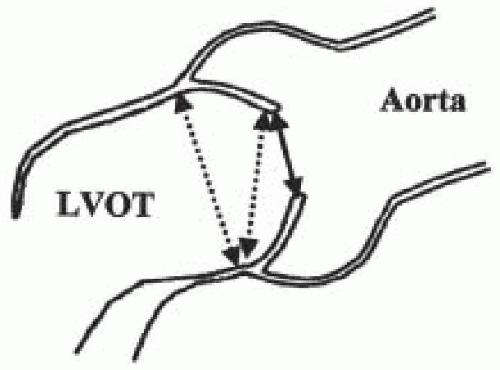
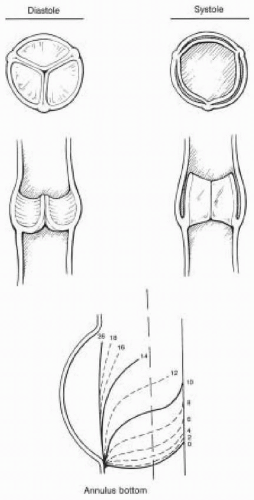
For patients undergoing valve replacement, another aspect of the focused exam is determination of valve size and suitability of the implantation of specific valve types. The midesophageal aortic valve long-axis view is used to measure annular diameter and size of the aortic root. A size discrepancy of greater than 10% between the diameters of the aortic annulus and sinotubular junction makes implantation of a stentless aortic valve unfeasible. The points at which the measurements are made are indicated in Fig. 30.3. The annular diameter measurement is the size used for implantation of mechanical and stented bioprosthetic valves, while the sinotubular junction diameter is the size used for implantation of stentless aortic valves.
Comprehensive Intraoperative Echo Exam
An accurate, comprehensive intraoperative echocardiography examination is paramount to the surgical decisionmaking process, particularly in patients in whom the severity of disease is borderline or moderate. The intraoperative echocardiography exam in these situations should utilize the full diagnostic potential of this tool and be performed by echocardiographers with advanced training (5). More than one method should be employed to assess the severity of disease, because the assessment will be used to guide the surgical decision. The severity of aortic stenosis can be determined by two-dimensional echocardiography, gradient determination by measurement of transaortic velocity, and area determination by planimetry and the continuity equation.
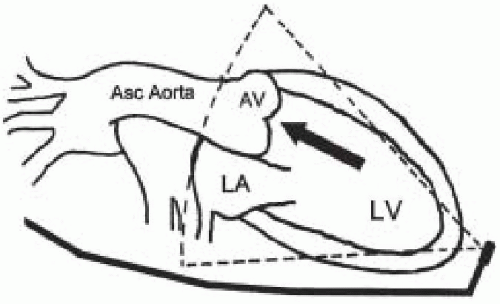
Hemodynamic assessment of antegrade and retrograde aortic valve flow requires a parallel orientation of
blood flow and the Doppler beam (Fig. 30.4). The midesophageal views used for two-dimensional evaluation are inadequate for this assessment because blood flow in these windows is perpendicular to the Doppler beam. Conversely, the two transgastric views are more useful for interrogating flow across the aortic valve, but not as useful for detailed two-dimensional anatomic assessment due to the far field position of the aortic valve. The deep transgastric long-axis (deep TG LAX) view is developed from the transgastric mid—short-axis view by advancing the probe past the midpapillary window, then gently withdrawing the probe with slight anteflexion and leftward flexion until the aortic valve is viewed in the middle or left side of the image in the far field (Fig. 30.5). The transgastric long-axis (TG LAX) view is developed from the transgastric mid—short-axis view by rotating the transducer forward from 0° to 90° to 120° until the aortic valve is viewed on the right side of the image in the far field (Fig. 30.6). Either one or both of the transgastric views can be used to measure the velocity of blood flow through the aortic valve and left ventricular outflow tract because of the parallel orientation of transaortic blood flow and the Doppler beam. It is important for the echocardiographer to become familiar with both approaches, because these views are often difficult to obtain and require considerable practice and expertise. Stoddard et al. demonstrated 56% feasibility among the first 43 patients studied as compared to 88% feasibility among the latter 43 patients studied, suggesting a significant learning curve in measuring aortic valve blood flow velocity via the transgastric approach (6). Data obtained intraoperatively is compared to preoperative assessment, and intraoperative surgical inspection of the valve can be used as a gauge of accuracy in aortic valve evaluation.
blood flow and the Doppler beam (Fig. 30.4). The midesophageal views used for two-dimensional evaluation are inadequate for this assessment because blood flow in these windows is perpendicular to the Doppler beam. Conversely, the two transgastric views are more useful for interrogating flow across the aortic valve, but not as useful for detailed two-dimensional anatomic assessment due to the far field position of the aortic valve. The deep transgastric long-axis (deep TG LAX) view is developed from the transgastric mid—short-axis view by advancing the probe past the midpapillary window, then gently withdrawing the probe with slight anteflexion and leftward flexion until the aortic valve is viewed in the middle or left side of the image in the far field (Fig. 30.5). The transgastric long-axis (TG LAX) view is developed from the transgastric mid—short-axis view by rotating the transducer forward from 0° to 90° to 120° until the aortic valve is viewed on the right side of the image in the far field (Fig. 30.6). Either one or both of the transgastric views can be used to measure the velocity of blood flow through the aortic valve and left ventricular outflow tract because of the parallel orientation of transaortic blood flow and the Doppler beam. It is important for the echocardiographer to become familiar with both approaches, because these views are often difficult to obtain and require considerable practice and expertise. Stoddard et al. demonstrated 56% feasibility among the first 43 patients studied as compared to 88% feasibility among the latter 43 patients studied, suggesting a significant learning curve in measuring aortic valve blood flow velocity via the transgastric approach (6). Data obtained intraoperatively is compared to preoperative assessment, and intraoperative surgical inspection of the valve can be used as a gauge of accuracy in aortic valve evaluation.
A common clinical dilemma facing clinicians who care for cardiac surgical patients is the patient with coronary artery disease (CAD) requiring coronary artery bypass grafting (CABG), who is found to have moderate aortic stenosis. Accepted practice for patients undergoing CABG with severe aortic stenosis (valve area < 1.0 cm2 and gradient > 40 mm Hg) is for combined aortic valve replacement (AVR) and CABG. Conversely, the consensus is CABG only for patients with CAD and mild aortic stenosis [aortic valve area (AVA) > 1.5 cm2 and aortic valve gradient (AVG) < 25 mm Hg]. Patients with moderate aortic stenosis (AVA of 1.0-1.5 cm2 and AVG of 25-40 mm Hg) undergoing CABG present a clinical controversy. Should
the patient with moderate aortic stenosis undergoing CABG have CABG alone or combined AVR and CABG?
the patient with moderate aortic stenosis undergoing CABG have CABG alone or combined AVR and CABG?
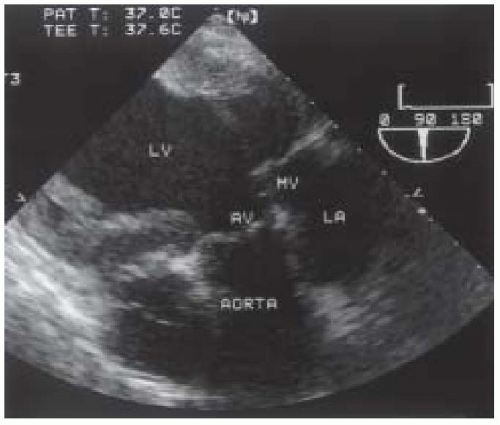 FIGURE 30.6. Transesophageal echocardiogram of the transgastric long-axis view. AV, aortic valve; AORTA, aortic root; LA, left atrium; MV, mitral valve; LV, left ventricle. |
The dilemma exists in patients with aortic stenosis that is not severe and often asymptomatic. These patients would not be candidates for AVR based on the severity of aortic stenosis alone, but because they are undergoing CABG surgery, consideration is made for concomitant aortic valve replacement. Aortic valve replacement after previous coronary artery bypass grafting is associated with higher mortality than combined aortic valve and coronary bypass surgery (7). It is therefore important to identify even moderate aortic stenosis during coronary bypass surgery and consider combination surgery to avoid the higher mortality associated with reoperation.
The degree of aortic stenosis is important for surgical management, because valves with a smaller valve area will progress more rapidly to cause symptoms related to aortic stenosis. The aortic valve short-axis view allows measurement of aortic valve orifice area by two-dimensional planimetry, which provides good correlation with other methods used for assessment of aortic stenosis (8). The probe is manipulated to provide the image with the smallest orifice to ensure that the cross section is of the leaflet tips. A cross section that is oblique or inferior to the leaflet tips overestimates the orifice size. The valve appears circular in a true short-axis cross section with all three cusps being viewed simultaneously and equal in shape. Multiplane TEE simplifies the location of the actual orifice by imaging the aortic valve, first in long axis to identify the smallest orifice at the leaflet tips. The orifice is centered on the image display screen and the transducer position is stabilized within the esophagus as the multiplane angle is rotated backward to the short-axis view. The smallest orifice is traced and the two-dimensional cross-sectional area is displayed (Fig. 30.8). Limitations to planimetry are:
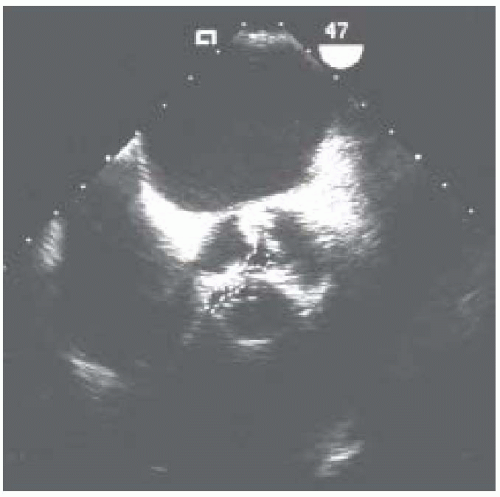 FIGURE 30.8. Transesophageal echocardiogram of the midesophageal aortic valve short-axis view during systole in a patient with aortic stenosis, using planimetry to measure aortic valve area. |
1. Inability to obtain an adequate short-axis view
2. Heavy calcification (particularly posterior), which causes shadowing of the valve
3. The presence of “pinhole” aortic stenosis, in which the valve orifice cannot be identified.
The presence of these elements suggests advanced disease or the likelihood of rapid progression of aortic stenosis, and favors a decision to replace the valve during concomitant CABG surgery.
Intraoperative echocardiography is not only critical in the evaluation of the aortic valve, but in the evaluation of cardiac and vascular structures that are affected by the techniques employed during aortic valve surgery. Evaluation of left ventricular function is important, because patients with aortic stenosis develop left ventricular hypertrophy, decreased left ventricular compliance, and are prone to myocardial ischemia. TEE provides early indication of myocardial ischemia through monitoring of regional wall motion, and a global assessment of overall contractility and volume loading. Patients with aortic stenosis have decreased left ventricular compliance; therefore assessment of preload using pulmonary artery catheter data often provides misleading information.
TEE provides a more accurate assessment of preload by thorough imaging of intracavitary volume and global contractility.
TEE provides a more accurate assessment of preload by thorough imaging of intracavitary volume and global contractility.
Patients with aortic valve disease develop diastolic dysfunction, which also can be evaluated and monitored with intraoperative echocardiography. Long-standing left ventricular dysfunction leads to mitral regurgitation, pulmonary hypertension, and ultimately right ventricular dysfunction. TEE is useful for managing these problems intraoperatively and for guiding surgical management decisions about the need for cardiac surgical intervention. For example, mitral regurgitation secondary to increased left ventricular systolic pressure from aortic stenosis will likely improve with aortic valve replacement. However, intrinsic mitral valve disease or mitral regurgitation due to hypertrophic obstructive cardiomyopathy may not improve after aortic valve replacement, and may in fact worsen.
Dilation of the ascending aorta may be a consequence of long-standing aortic stenosis due to the body’s adaptive mechanism in promoting left ventricular ejection, or may be secondary to intrinsic disease within the aortic walls. The latter is more likely to require surgical intervention, while the former does not require surgical correction unless the dilation is severe. Evaluation of the aorta is also important for determining the cannulation site and for guiding perfusion strategies, in addition to identifying atheromatous disease in the proximal ascending aorta, implicated as a leading cause of neurologic injury.
Postoperatively, the comprehensive examination is focused on the function of the prosthetic valve, postimplant complications, and resolution of dynamic lesions not surgically addressed. Postbypass echocardiography is particularly important for patients undergoing valve repair to assess the success of repair. The presence of aortic regurgitation after aortic valve repair is a poor prognostic indicator, as these patients commonly require reoperation for definitive correction.
AORTIC VALVE APPARATUS
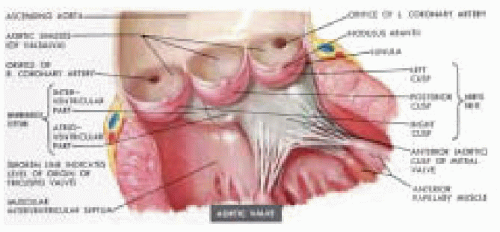
Understanding the role of intraoperative echocardiography in aortic valve surgery requires knowledge of the anatomy and function of the aortic valve apparatus. The leaflets and the supporting and surrounding structures constitute the aortic valve apparatus (Fig. 30.9). Akin to the mitral valve apparatus being comprised of chordae tendineae, papillary muscles, and the annulus, the aortic valve apparatus is comprised of the valve cusps, the sinuses of Valsalva, the proximal ascending aorta, and the left ventricular outflow tract. Abnormalities involving any of these structures can lead to aortic valve dysfunction. Proximal to the aortic valve, the left ventricular outflow tract consists of the inferior surface of the anterior mitral leaflet, the ventricular septum, and the posterior left ventricular free wall.
The pressure drop across the aortic valve during diastole generates considerable stress within the leaflets. An intact apparatus allows distribution of this stress from the leaflets to the surrounding fibrous structure to which the leaflets are attached (9). The leaflets are also supported by one another along the region of coaptation on the leaflet called the lunula (Fig. 30.9). The stress is then distributed along the leaflet edges to corners of the commissures (9). Further stress reduction occurs through distribution of stress to the sinuses of Valsalva, which are three bulges or pouch-like dilations in the aortic wall associated with each leaflet (Fig. 30.9). The radius of curvature within these bulges in the aortic wall decreases between systole and diastole to accommodate the stress in accordance with LaPlace’s law (9,10). The sinuses not only play an important role in leaflet closure by distributing stress, but their presence also prevents the leaflets from making contact with the aortic wall during systole. All of these components of the aortic valve apparatus are important for the proper functioning and durability of the valve. Any disruption of these mechanisms within the apparatus will lead to valve dysfunction and premature deterioration. Understanding these mechanisms is essential for the surgeon repairing these valves and to the echocardiographer who must interpret echocardiographic findings associated with valve dysfunction and communicate these findings to the surgeon.
A normal aortic valve appears as two thin lines that open parallel to the aortic walls in the midesophageal aortic valve long-axis (ME AV LAX) view. This view also provides imaging of the ascending aorta and LVOT (Fig. 30.10) and provides valuable insight into the function of the aortic valve apparatus. With atrial contraction and left ventricular filling during late diastole, a 12% expansion of the aortic root is observed 20 to 40 milliseconds prior to
aortic valve opening (9,11,12). Dilation of the aortic root alone contributes 20% to leaflet opening (9). The effect of this aortic root dilation actually causes the leaflets to open before any positive pressure from ventricular contraction is applied (9,13). As previously mentioned, leaflet stress during diastole is distributed along the leaflet edges, to the commissures, and to the aortic root. Just before the onset of systole, with no tension within the leaflets and the aortic root dilated, the leaflets open rapidly with the onset of ventricular contraction, offering minimal resistance to left ventricular ejection (9,14).
aortic valve opening (9,11,12). Dilation of the aortic root alone contributes 20% to leaflet opening (9). The effect of this aortic root dilation actually causes the leaflets to open before any positive pressure from ventricular contraction is applied (9,13). As previously mentioned, leaflet stress during diastole is distributed along the leaflet edges, to the commissures, and to the aortic root. Just before the onset of systole, with no tension within the leaflets and the aortic root dilated, the leaflets open rapidly with the onset of ventricular contraction, offering minimal resistance to left ventricular ejection (9,14).
The sinuses of Valsalva play an important role during systole, allowing the leaflets to fully open without making contact with the aortic wall. In addition, because a space is maintained between the leaflets and the aortic wall, the leaflets rapidly close at the onset of diastole (Fig. 30.11). The sinuses provide a reservoir of blood for developing vortices that move toward the ventricular arterial junction as the velocity of blood flow declines during systole (9). These vortices within the sinuses of Valsalva prime the leaflets for closure, so that as soon as the pressure between the ventricle and aorta equalizes, the leaflets rapidly close.
PATHOLOGY
Pathophysiology of Aortic Stenosis
The most frequent causes of aortic stenosis are calcific stenosis in the elderly, rheumatic valvulitis, and congenital anomalies (bicuspid, rarely unicuspid) that lead to accelerated leaflet calcification and restriction. The mechanism of stenosis occurs from calcification of the leaflets (calcific, rheumatic, or congenital) or commissural fusion (usually rheumatic). Subaortic stenosis (subaortic membrane or ridge, and asymmetric septal hypertrophy) and supravalvular stenosis (narrowed aortic root) mimic aortic stenosis, but do not represent true valvular stenosis.
Many of the echocardiographic techniques used for hemodynamic assessment of the aortic valve, however, can also be used to evaluate the severity of subvalvular and supravalvular pathology. Asymmetric septal hypertrophy or hypertrophic obstructive cardiomyopathy is further discussed in Chapter 15.
Many of the echocardiographic techniques used for hemodynamic assessment of the aortic valve, however, can also be used to evaluate the severity of subvalvular and supravalvular pathology. Asymmetric septal hypertrophy or hypertrophic obstructive cardiomyopathy is further discussed in Chapter 15.
Echocardiographic Evaluation of Aortic Stenosis
An important sign of aortic stenosis is leaflet doming during systole observed in the midesophageal long-axis view with two-dimensional echocardiography (Fig. 30.2). The leaflets are curved toward the midline of the aorta instead of parallel to the aortic wall. Leaflet doming is such an important observation that this finding alone is sufficient for the qualitative diagnosis of aortic stenosis. Coincident with doming is reduced leaflet separation (< 15 mm), which is appreciated in both the short- and long-axis views of the aortic valve. The short-axis view of the aortic valve (Fig. 30.1) permits evaluation of leaflet motion and calcification, commissural fusion, and leaflet coaptation. This short-axis view (30° to 60°) is used for measuring the aortic valve orifice area by two-dimensional planimetry, which provides good correlation with other methods used for assessment of aortic stenosis (8). The probe is manipulated to provide the image with the smallest valvular cross-sectional area to ensure that the imaging plane is at the level of the leaflet tips or smallest orifice.
The two-dimensional evaluation of the aortic valve is usually sufficient to confirm the diagnosis of aortic stenosis. Patients with a diagnosis of moderate aortic stenosis, who are not predetermined to undergo an aortic valve replacement, require more sophisticated evaluation of their aortic valve disease. Doppler echocardiography is used to quantitate the severity of aortic stenosis by measuring transvalvular blood velocity. The peak pressure gradient is estimated from the peak velocity measurement using the modified Bernoulli equation:
Aortic Valve Gradient = 4 × (Aortic Valve Velocity)2
The deep TG LAX view is used to direct the ultrasound beam in a parallel alignment with blood flow in the LVOT and through the aortic valve (Fig. 30.5). A parallel alignment of the ultrasound beam and aortic valve flow can also be obtained with the TG LAX view (Fig. 30.6).
The continuous wave Doppler cursor is aligned with the narrow, turbulent, high-velocity jet and the spectral Doppler display is activated. Accurate localization provides a distinctive audible sound and high-velocity (> 3 m/sec) spectral Doppler recording that exhibits a fine feathery appearance and a midsystolic peak (Fig. 30.12). Planimetry of the velocity over time spectral Doppler analysis of transaortic blood flow yields the velocity time integral (VTI) and an estimate of mean aortic valve gradient.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree